- 1State Key Laboratory of Plant Genomics, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
- 2CAS Center for Excellence in Biotic Interactions, University of Chinese Academy of Sciences, Beijing, China
- 3Beijing Institutes of Life Science, Chinese Academy of Sciences, Beijing, China
Insect vector-borne diseases are a major constraint to a wide variety of crops. Plants integrate environmental light and internal signalings to defend dual stresses both from the vector insects and vector-transmitted pathogens. In this review, we highlight a studies that demonstrate how light regulates plants deploying mechanisms against vector-borne diseases. Four major host defensive pathways involved in the host defense network against multiple biotic stresses are reviewed: innate immunity, phytohormone signaling, RNA interference, and protein degradation. The potential with light-engineering technology with light emitting diodes (LEDs) and genome engineering technology for fine-tuning crop defense and yield are also discussed.
Introduction
Global warming has driven the emergence and reemergence of insect vector-borne plant diseases soaring and causes a huge loss in agricultural production. These microbial pathogens and parasites, especially viruses and bacteria, are transmitted by arthropod vectors. Efforts to control these diseases have been emphasized on the use of chemical pesticides. Pesticide abuse has resulted in pesticide resistance on these extremely polyphagous arthropod species, via either physiological, biochemical, or behavioral mechanisms. Especially for viruses, approximately 80% of 1,480 known plant viruses are arthropod vector transmitted and cause billions of dollars loss annually (Ye et al., 2021). The outspreading diseases caused by insect-transmitted plant viruses in the past decades have been mainly driven by planthoppers, whiteflies, aphids, and thrips (Dáder et al., 2015; Wu and Ye, 2020). Besides chemical measures, both the biological and physical strategies, such as light quality and quantity, have recently been highlighted for controlling plant viral diseases (Montes and Pagán, 2019; Zhai et al., 2020; Gallé et al., 2021).
Numerous pieces of evidence have demonstrated that different kinds of light function as antimicrobial and antiviral therapies against human bacterial and viral diseases. Violet/blue light accounts for the Nobel Prize in 1903 given to Niels Ryberg Finsen for the successful treatment of tuberculosis caused by Mycobacterium tuberculosis. In a similar way, the potential reduction of phytopathogenic infections in plants could be brought out by the ubiquity of inexpensive light emitting lasers or light emitting diodes (LEDs), which makes it easier to develop safe and low-cost devices. Balancing the effects of light spectrum on crop growth and crop protection is, therefore, required for optimal crop production and quality. The function of light in regulating plant abiotic stress responses, such as temperature responses and drought resistance, to maintain the normal growth and development of plants has been widely reported (D’Amico-Damião and Carvalho, 2018; Szalai et al., 2018; Roeber et al., 2021; Wang et al., 2021a). In this review, we address fundamental aspects of plant responses to supplemental light of specific wavelengths from the perspective of developing greenhouse crop production. We focus on the herbivorous insect as biotic stress because their behavior, as well as the behavior of their natural enemies, may also be affected by supplemental light. The effects of additional far-red (FR), red, blue, and UV components of the light spectrum on ambient greenhouse conditions fundamentally change host plant traits including disease resistance. Although we focus on the effects of supplemental LED light of specific wavelengths, we note that similar results may be obtained with other artificial light sources that also provide specific wavelengths.
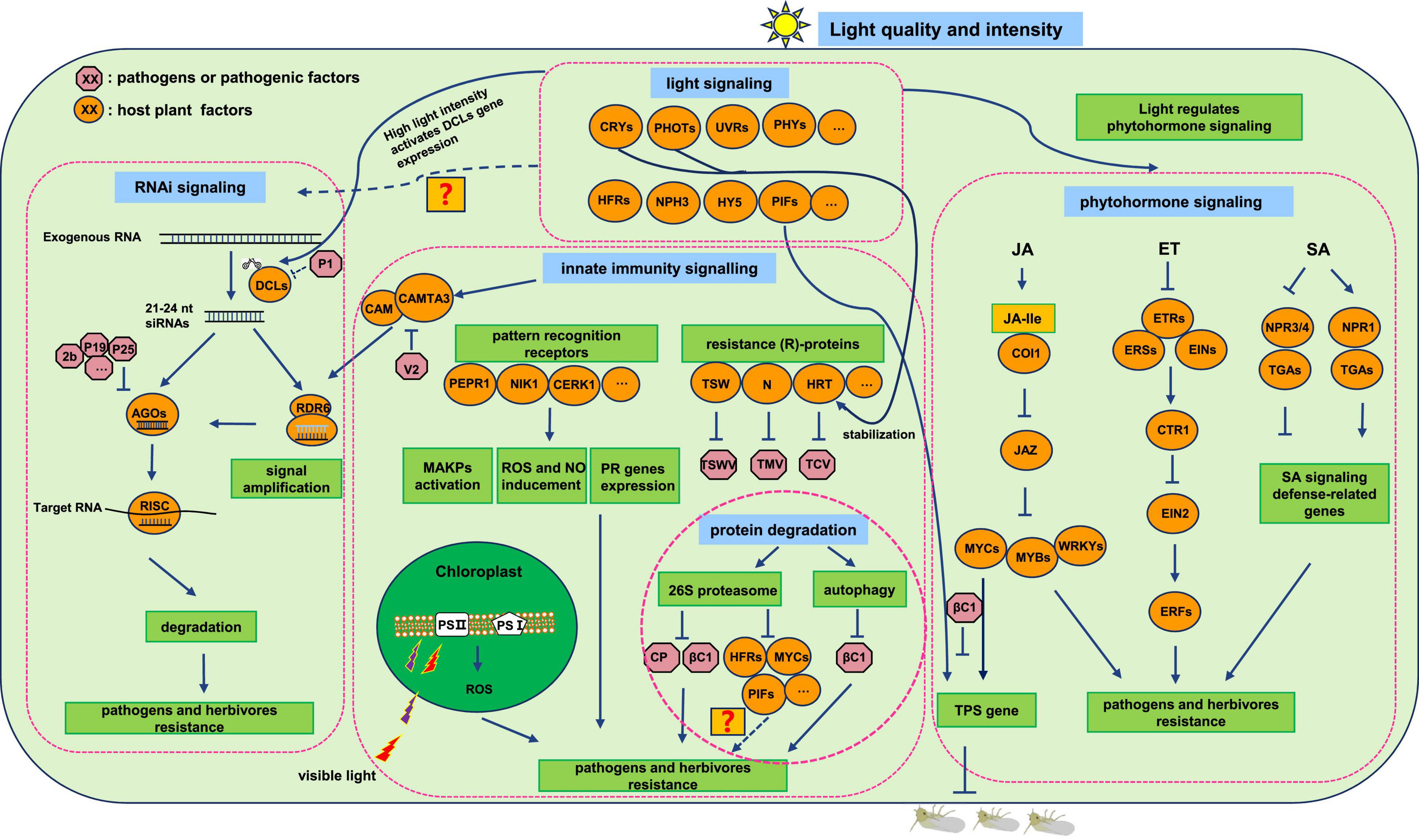
Instead of directly killing pathogens, environmental light regulates plant resistance to defend against the invasion of pathogens. Plants have evolved multilayered defense mechanisms including innate immunity, hormone signaling, autophagy and/or 26S proteasome-mediated protein degradation, and RNA interference (RNAi) in plant-pathogen interactions. Insect-borne pathogens are highly dependent on their insect vectors during their natural transmission cycle. Tripartite interaction study among plant–pathogen–insect is more complicated compared with bipartite interactions including plant–pathogen and plant-insect. Understanding plant defensive responses upon multiple stresses simultaneously are of greater significance for developing efficient disease-control strategies. In this review, we mainly summarized the recent advancements in plant defensive pathways, mainly containing plant innate immune response, hormone signaling pathways, RNAi, and protein degradation pathways, combined with the regulation of light signaling toward these resistance pathways.
Light Signaling Pathway in Plants
Light is indispensible for the growth and stress responses in the whole life of plants since it is the only energy source in a form of electromagnetic radiation from solar. Likewise, light has properties of both the waves and particles and is able to induce DNA damage and other plant stress responses. Photosynthesis in chloroplasts, which is believed to be descended from a prokaryotic ancestor, is one main way for plants to perceive light signals. Photosynthetic processes in plants mainly absorb a photon and use visible light with wavelengths of 400–700 nm. Not all the wavelengths of lights have equal energy and the energy content of light is inversely proportional to its wavelength. Excess and fluctuating light results in reactive oxygen species (ROS) accumulation around photosystems II and I, respectively. ROS accumulation leads to broad-spectrum tolerance against both the abiotic and biotic stresses (Kangasjärvi et al., 2014; Liu et al., 2019; Roeber et al., 2021). Besides photosynthesis, plants sense light in a second way with a series of photoreceptors located in the cytoplasm and nucleus as well as their downstream factors that function in light sensing and signal transduction. Plant photoreceptor-mediated light signalings play fundamental roles in plant growth and defensive responses.
At least five classes of photoreceptors sense unique light wavelengths (Paik and Huq, 2019; Roeber et al., 2021). Phytochromes (PHYS), which mainly sense red and FR light (600–750 nm), contain phyA-phyE five receptors in Arabidopsis thaliana. Cryptochromes (CRYs), encoded by CRY1 and CRY2, sense blue, green, and UVA light (320–500 nm) (Folta and Maruhnich, 2007; Liu et al., 2016). Phototropins (PHOTs) (known as PHOT1 and PHOT2) as well as ZEITLUPE (ZTL)/FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1)/LOV KELCH PROTEIN2 (LKP2) are illustrated as blue-light receptors (Christie, 2007; Zoltowski and Imaizumi, 2014). The last type of recognized photoreceptor is UV RESISTANCE LOCUS 8 (UVR8), which senses UVB radiation (280–320 nm) (Demkura and Ballaré, 2012; Heijde and Ulm, 2012; Liang et al., 2019). Once the various light signals are detected by these receptors respectively, plants will initiate the downstream responses to regulate their growth, development, and immunity. These photoreceptors regulate either the core factors of the common pathway such as CONSTITUTIVE PHOTOMORPHOGENIC 1/SUPPRESSOR OF PHYA1 (COP1/SPA1) complex or distinctive branches of their sensed signals to mediate light perception responses of plants. COP1/SPA1 complex is an E3 ubiquitin ligase and is usually considered as a negative regulator of photoreceptors regulated light responses which is by degradation of numerous positive transcription factors of light signaling pathways such as ELONGATED HYPOCOTYL 5 (HY5), LONG AFTER FAR-RED LIGHT 1 (LAF1), LONG HYPOCOTYL IN FAR-RED 1 (HFR1), and so on (Lau and Deng, 2012).
Phytochromes (such as phyB) can directly interact with the COP1/SPA complex and interfere with its function in HY5 degradation, which ultimately contributes to photomorphogenesis (Hoang et al., 2019; Li et al., 2020). Except for this indirect regulation of negative factors for photomorphogenesis, phytochromes are also able to directly interact with phytochrome-interacting factors (PIFs) and inhibit their roles in photomorphogenesis repression by phosphorylation and polyubiquitylation-mediated degradation pathway (Park et al., 2004; Shen et al., 2005, 2008; Al-Sady et al., 2006). Through indirect and direct interactions with these master factors in the light pathway, phytochromes regulate the whole living life of plants from seed germination, photomorphogenesis to flowering time, as well as shade avoidance, circadian clock, gravitropism, and even more importantly, the defense responses (Correll et al., 2003; Casal, 2013; Pierik and de Wit, 2014; Roig-Villanova et al., 2019; Fernández-Milmanda and Ballaré, 2021; Roeber et al., 2021). Cryptochrome-mediated signal transduction is divided into different ways through interacting with various proteins of plants, for example, the CRY/COP1/SPA complexes, cryptochrome-interacting basic helix-loop-helix (CRY/CIB) complexes, CRY/PIF complexes, and so on (Jiao et al., 2007; Liu et al., 2011; Wang and Lin, 2020). Another photoreceptor that regulates the transcription factors of light signaling through targeting the COP1/SPA1 complex is UVR8 for stabilization of HY5 to initiate UVB-mediated gene expression (Cloix et al., 2012; Huang et al., 2013). On the other hand, UVR8 could directly interact with BRI1-EMS-SUPPRESSOR1 (BES1); BES1-INTERACTINGMYC-LIKE1 (BIM1) and WRKYDNA-BINDINGPROTEIN36 (WRKY36). to function in photomorphogenesis (Liang et al., 2018; Yang et al., 2018a). ZTL/FKF1/LKP2 family proteins transduce blue light signals primarily by altering the activity of the Skp1-CUL1-F-boxprotein (SCF) E3 ligase complex, which mediates the SCF E3 ligase targeted proteins degradation for circadian clock and photoperiodic flowering regulation (Song et al., 2014; Zoltowski and Imaizumi, 2014). Phototropin is also a well-known blue light photoreceptor; it has been reviewed that PHOT1 could interact with NON-PHOTOTROPIC HYPOCOTYL 3 (NPH3) and PHYTOCHROME KINASE SUBSTRATE 4 (PKS4) to elicit photomorphogenic responses (Demarsy et al., 2012). These photoreceptors sense distinguished light spectrum for orchestrating the signaling transductions of plants such as hormone signaling to regulate plant growth, development, and defense responses. The next part will focus on the plant defense responses regulated by light signaling.
Light Regulated Plant Innate Immunity Against Insect-Borne Pathogens
The innate immune response is a well-studied defense pathway in plants. The classical defense and counter defense responses between plants and pests or pathogens are based on the “herbivore-/microbe-associated molecular patterns (HAMPs/MAMPs) to pattern-triggered immunity (PTI)” and “herbivore-/microbe-derived effectors to effector-triggered immunity (ETI)” (Zhou and Zhang, 2020; Ye et al., 2021). Relying on these two immune pathways, plants cannot only recognize but also resist insects and pathogens. Although the latest studies reveal that there may not be a clear boundary between plant PTI and ETI as the cooperation of these two pathways for promoting resistance, while the defense responses of PTI and ETI are usually considered to be different from each other (Ngou et al., 2021; Yuan et al., 2021). PTI acts as a basal immune response usually recognizes pathogens elicitors by pattern recognition receptors (PRRs) localized on the cell surface (such as cell walls and cell membranes), which triggers relatively mild defensive responses, such as ROS and nitric oxide (NO) inducement, mitogen-activated protein kinases (MAPKs) activation, phytohormones regulation, callose deposition, and pathogenesis-related (PR) genes expression, which ultimately inhibits non-adapted microbes to infect plants (Bigeard et al., 2015; Waheed et al., 2021). While ETI acts as a secondary response commonly recognizes pathogen effectors by intracellular localized resistance (R) proteins, which can produce robust defensive responses, such as hypersensitive response (HR; Balint-Kurti, 2019; Waheed et al., 2021).
Accumulating reports are complementing and perfecting the blueprint of plant innate immune pathways to fight against invaders. Higher plants have evolved a series of cell surface and intracellular immune receptors for sensing and resisting pathogen infections and herbivore infestations. Different types of PRRs have been identified including leucine-rich repeat (LRR), lysine motifs (LysMs) containing receptor proteins, and lectin-type PRRs binding extracellular ATP or bacterial lipopolysaccharides (LPSs; Ye et al., 2021). The functions of these PRRs that recognize elicitors from pathogenic organisms such as bacterial flagellin, fungal chitin, and herbivorous fatty acid–amino acid conjugates (FACs) were largely reviewed by numerous excellent articles (Boutrot and Zipfel, 2017; Saijo et al., 2018; Abdul Malik et al., 2020). R proteins functions in ETI are mainly intracellular nucleotide-binding (NB) LRR domain receptors (NLRs), which recognize specific effectors derived from pathogens or pests including genes such as N gene from tobacco to tobacco mosaic virus (TMV), Sw5b from tomato as well as Tsw from pepper to tomato spotted wilt orthotospovirus (TSWV; Erickson et al., 1999; Zhu et al., 2019). In consistence with the interaction model of plant-insect, plant–bacteria, and plant–fungi in the innate immune pathway, no conserved elicitor from the virus has been found, although many reports indicate that viruses can trigger PTI and ETI responses in plants and many virus-encoded proteins are considered to be the most important suppressors to counter plant defenses. Therefore, the plant defense response to the virus has its unique classical pattern such as RNAi. Actually, it is considered by researchers that RNAi works as PTI to recognize virus-derived elicitors small RNAs and is suppressed by viral effectors (Zvereva and Pooggin, 2012; Nakahara and Masuta, 2014). Cases and mechanisms involved in plant innate immune responses to fight against insect-borne pathogens by PTI and ETI have been broadly reported and summarized. An interesting study revealed a whitefly-transmitted begomovirus cotton leaf curl Multan virus (CLCuMuV), which encoded a pathogenic factor βC1 by its associated betasatellite that targeted a newly identified key factor of plant immune response pathway, WRKYDNA-BINDINGPROTEIN20 (WRKY20) transcription factor, to redeploy plant chemical immunity within the leaf for benefiting virus whitefly vectors while negatively affecting two non-vector competitors (Zhao et al., 2019). With the deepening of research, it will be found that the defense and counterdefense between plants and attackers are becoming more and more complex. These previous studies will promote us to research more complicated interaction systems and scarcely studied pathogens, which cause huge damage to agriculture. For example, Huanglongbing, the causal agent of it is citrus psyllid-transmitted Candidatus Liberibacter asiaticus (CLas), is an intractable plant disease that limits the yield of citrus. Due to the unculturable and phloem-restrictive characteristics, studies of CLas face great challenges (Ferrarezi et al., 2020). Therefore, exploring the elicitors and effectors from CLas and its insect vector-citrus psyllid have significant roles in finding effective strategies against Huanglongbing disease.
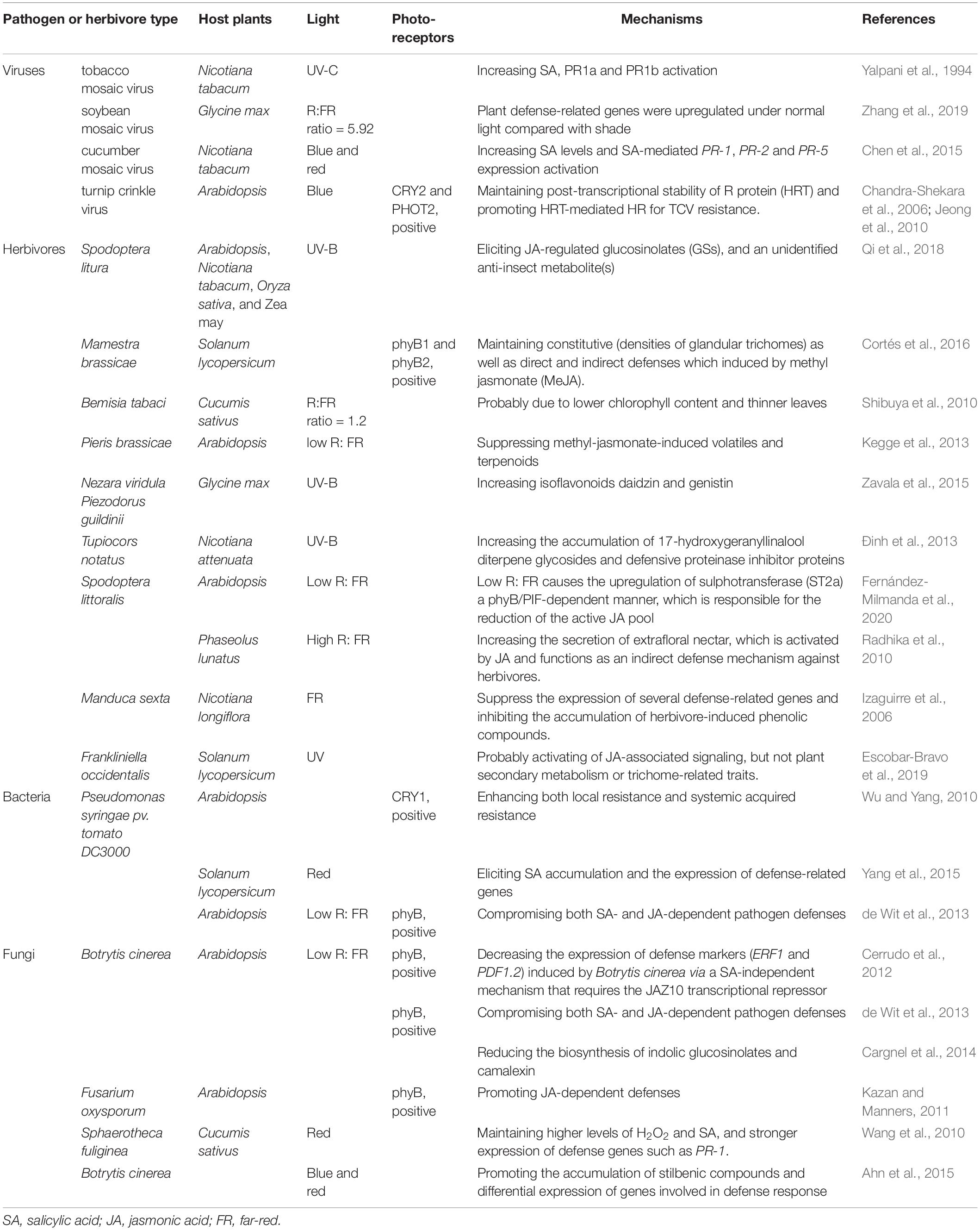
Tremendous reports demonstrated the essential roles of a certain spectrum of light in promoting plant defense against pathogen infection and herbivore infestation. Normally, red light increases plant resistance to various pathogens, herbivores, and nematodes (Yang et al., 2018b; Gallé et al., 2021), although the molecular mechanisms were still obscure. As described above, plant innate immunity is an essential strategy deployed by plants to counter invaders. The light could regulate plant resistance to pathogens through manipulating the plant innate immunity pathway, especially for the R gene regulated resistance. Wu and Yang (2010) showed that blue light photoreceptor CRY1 is involved in promoting R protein-mediated plant resistance to Pseudomonas syringae pv. tomato DC3000 carrying avrRpt2 in Arabidopsis. The effector-triggered local resistance and systemic acquired resistance (SAR) were both impaired in the cry mutant and salicylic acid (SA)-induced PR gene PR-1 expression is reduced as well. Despite the light-mediated R gene resistance to bacteria, a similar agent also occurred in an insect-borne virus. Chandra-Shekara et al. (2006) demonstrated that light was required for R protein hypersensitive response to TCV (HRT)-mediated HR and resistance to turnip crinkle virus (TCV). Jeong et al. (2010) further showed that blue-light photoreceptors, CRY2 and PHOT2, maintained post-transcriptional stability of HRT, thereby resisted TCV. These studies unrevealed the resistance regulated functions of essential factors in light signaling, which provides a significant insight into the further exploration of light signaling-mediated plant defense against insect-borne pathogens.
Light-Mediated Phytohormone Signaling Against Insect-Borne Pathogens
Hormone signaling pathways regulate plant growth, development, and defense responses in their whole life. Among the various phytohormones, SA, jasmonic acid (JA), and ethylene (ET) are widely reported to be resistant to biotic stresses. Generally speaking, SA is usually considered as a primary hormone against biotrophic, hemibiotrophic pathogens, and phloem-feeding insects; JA and ET mainly regulate plant immune responses against chewing insects and necrotrophic pathogens (Lazebnik et al., 2014; Li et al., 2019; Gallé et al., 2021). Understanding how plants coordinate phytohormone signaling to counteract serious external threats makes great sense for exploring novel disease resistance strategies.
Jasmonic acid signaling is initiated by the generation of jasmonoyl-L-isoleucine (JA-Ile), once plants perceive stimuli from the external environment. Then, JA-Ile binds to F-box protein CORONATINE INSENSITIVE1 (COI1) to degrade JAZ through the 26S proteasome pathway for releasing JASMONATE ZIM-DOMAIN PROTEIN (JAZ) inhibited downstream genes expression of JA signaling such as transcription factor (TF) families of MYCs, MYBs, WRKYs, etc., (Ruan et al., 2019; Wu and Ye, 2020). These TFs regulate plant defense responses to pathogens and herbivores by activating the resistance genes expression. For instance, terpene synthase (TPS) genes for synthetizing TPS, whose products could regulate herbivore appealing or avoiding (Tholl, 2015; Chen et al., 2020). Vegetative storage proteins (VSPs) genes encoded toxic proteins mainly induce defensive responses to fight against insect herbivores (Schweizer et al., 2013; Wu and Ye, 2020). Multiple articles have demonstrated the resistance roles of JA signaling in plant-pathogen interactions, especially when concerned with the complicated tripartite interactions, which usually occur when vector-borne pathogens infect plants. For example, begomovirus tomato yellow leaf curl China virus (TYLCCNV)-associated betasatellite encoded βC1 hijacks the core factors, such as JA signaling pathway MYC2 and light signaling pathway PIFs, to repress their transcription factor activities, which mediates TPS gene expression for promoting the attraction of virus vector-whitefly (Li et al., 2014; Zhao et al., 2021). Except for begomoviruses, which belong to the DNA virus, the vector-borne RNA virus was also reported to repress JA signaling for promoting their vector performance. TSWV, which is transmitted by thrips, encodes a nonstructural-protein (NSs) for interacting with and disturbing the function of MYC2/3/4 to disable JA-mediated activation of TPS genes. This modifies host volatiles and increases vector preference, which ultimately promotes vector performance in the host (Wu et al., 2019).
Salicylic acid signaling has profound importance in regulating plant resistance against biotrophic, hemibiotrophic pathogens, and some phloem-feeding herbivores, which usually cause minor damage to plants (Onkokesung et al., 2016; Li et al., 2021). Two classes of receptors, NONEXPRESSER OF PR GENES1 (NPR1) and NPR3/NPR4, perceive SA, although they play opposite roles in regulating defense gene expression (Figure 1; Zhang and Li, 2019; Zhou and Zhang, 2020). NPR1, as the transcription activator in SA signaling, functions in sensing SA and repressing the transcriptional inhibition activities of NPR3/NPR4, which promotes the expression of defense-related genes, PR genes (Liu et al., 2015; Ding et al., 2018). SA plays a significant role in plant SAR, which is considered as an essential pathway for disease resistance. A growing number of researchers have reported the SA signaling-mediated plant resistance to insect-borne pathogens, which will fulfill our understanding of plant disease defense strategies and promote the process of exploration of disease-resistant cultivars.
Ethylene is well-known for regulating plant growth, fruit ripening, and stimulation of seed germination. Although ET signaling also plays a pivotal role in plant defense responses, the related cases are not widely reported as for JA and SA signaling. ET signaling initiates through ET perceiving by membrane-localized receptor dimer protein kinases such as ETHYLENE RESPONSE1/2 (ETR1/ETR2), ETHYLENE RESPONSE SENSOR1/2 (ERS1/ERS2), and ETHYLENE INSENSITIVE4 (EIN4), which releases the inhibition of these receptors to ET signaling followed by a series of protein activation and degradation processes, and then ET signaling-responsive genes express to regulate plant growth and resistance (Figure 1; Ju and Chang, 2015; Waadt, 2020). The TFs of the ethylene response factor (ERF) family are found to regulate plant resistance to a variety of pathogens and insects. For example, ERF3 identified in rice (Oryza sativa) positively affects gene expression of trypsin proteinase inhibitors as well as mediates resistance toward Chilo suppressalis caterpillars (Lu et al., 2011). This study provides a novel breeding target for plants to resist insects as well as their transmitted pathogens.
Light-mediated phytohormone signaling plays significant roles in regulating plant defense responses, as it was proved by various articles that a series of monochromatic light, such as red light, blue light, and UV light, displayed plant defense enhancement functions by activating phytohormone signaling pathways (Ballaré, 2014). Moreover, it is generally accounted for the red light, some reports also recorded that blue light and UV light could enhance plants’ SA and JA signaling, whereas FR or low R:FR ratio compromised these signaling pathways (Ballaré, 2014). For instance, red light illumination overnight enhanced host resistance against Pseudomonas syringae pv. tomato DC3000 (Pto DC3000), as this treatment elicited SA accumulation and the expression of defense-related genes in tomato (Solanum lycopersicum L.) plant leaves (Yang et al., 2015). Despite the resistance to bacterial pathogens, red light also contributes to defending against fungi such as broad beans (Vicia faba L.) infecting Botrytis cinerea, rice infecting Magnaporthe grisea, and cucumber infecting Sphaerotheca fuliginea. The resistance pathways activated by a red light to defend these fungi are via influencing hydrogen peroxide (H2O2), ascorbate peroxidase (APX), and catalase (CAT) enzyme activities (Ueno et al., 2007; Wang et al., 2010; Ahn et al., 2015). Moreover, red light also induces systemic resistance to fight against root-knot nematode by coordinating regulation of SA, JA, and redox signaling in watermelon (Yang et al., 2018b). Red light-mediated defense response to fight against the insect-borne virus through phytohormone signaling was also reported. In this case, SA levels and SA-mediated PR-1, PR-2, and PR-5 expression in Nicotiana tabacum (N. tabacum) were increased by red light treatment and these responses effectively delayed symptom expression and replication of cucumber mosaic virus (CMV) on N. tabacum, notably blue light treatment made the same effect as red light treatment in this study (Chen et al., 2015). In addition to CMV, TMV was also reported to be defended by the host through UV light eliciting SA signaling (Yalpani et al., 1994). Ðinh et al. (2013) have reported that UVB increased phytohormones accumulation of JA, JA-Ile, and abscisic acid (ABA), which play important roles in regulating plant defense against the biotic and abiotic stresses. Despite these lights eliciting phytohormone-regulated resistance, FR or low R:FR ratio was generally commended as a negative regulator for increasing phytohormone-regulated responses and tremendous studies have illustrated the attenuated SA- and JA-dependent defense responses against various attackers from pathogens to herbivores (Table 1). These studies describe the essential functions of light with specific wavelength in regulating plant hormone pathways to resist pathogens, though the detailed molecular mechanisms are unclear, especially the essential host plant regulators involved and how pathogens or insects evolved to escape host defense.
Genetic experiments confirmed the important roles of photoreceptors in light-mediated resistance responses with indication phyB mutants of various species of plants showing increased sensitivity to herbivores and pathogens (Cortés et al., 2016; Courbier et al., 2020). These studies demonstrated that red light-mediated plant defense response was initiated along with the perception of red light by photoreceptor phyB and then phyB primed downstream resistance responses. Actually, phyB inactivation destabilizes MYC stability in a COP1-dependent manner (Chico et al., 2014). In addition, inactivating phyB causes more available JAZ10, a negative regulator of JA signaling, which attenuates JA signaling-mediated defense responses (Leone et al., 2014). These studies indicated that red light regulated protein stabilities of plant hormone pathways through its photoreceptor to orchestrate plant defense responses to fight against attackers. While red light enhanced plant defense is not suitable for all the interaction cases between plants and pathogens, especially when concerned with multiple interactions and specific pathogens. A recent study about red light-mediated tripartite interaction of plant-begomovirus-whitefly demonstrated the beneficial effects of red light on both the whitefly and the begomovirus. Zhao et al. (2021) found that red light promoted the mutualism of whitefly-begomovirus by stabilizing βC1 protein encoded by TYLCCNV-associated betasatellite and accumulated βC1 further inhibits PIFs positively controlling of plant defenses against whitefly by reducing the promoter-binding activity of PIFs to TPS genes. Furthermore, βC1 also decreased the transcriptional activity of PIFs and MYC2 via disturbing their dimerization, thus impairing plant defenses against TYLCCNV transmitted vector-whitefly.
Light-Mediated RNA Interference Signaling Pathway Against Insect-Borne Pathogens
Ribonucleic acid interference (RNAi) (also called RNA silencing) is involved in broad regulative pathways with nucleotide sequence specific, and it is mediated by small RNAs. The small RNAs include microRNAs (miRNAs), short-interfering RNAs (siRNAs), PIWI-related RNAs (piRNAs), and so on. Unlike piRNAs, which are only found in animals, miRNAs and siRNAs are found in most eukaryotes and function as the primary factors for guiding the antiviral immune process in plants (Martínez de Alba et al., 2013; Muhammad et al., 2019; Niehl and Heinlein, 2019). In addition to the three types of small RNAs for priming RNAi, three core families of proteins for achieving RNAi-involved defense are indispensable equally such as Dicer-like (DCL) protein, RNA-dependent RNA polymerase (RDR), and argonaute (AGO) protein. These proteins coordinate together to fine-tune plant resistance to pathogens and herbivores. As a counterdefense strategy, pathogens employ their multifunctional proteins as suppressors to defend against RNAi by targeting the essential proteins in this pathway. So far, large numbers of viral suppressors of RNAi (VSRs) have been characterized from all the plant virus families. The main antagonize mechanism employed by these VSRs is to interfere with the various steps of the RNAi pathway (Li and Ding, 2006; Song et al., 2011). For example, VSRs of diverse plant viruses suppress siRNA production, siRNA sequestration, and systemic silencing. The typically representative VSRs are Potyvirus helper component-proteinase (HC-Pro), cymbidium ringspot virus (CymRSV) P19, and potato virus X (PVX) P25, respectively (Voinnet et al., 2000; Mallory et al., 2002; Lakatos et al., 2004). The development of protein-protein interaction experiments has identified many key players of the plant RNAi pathway targeted by VSRs as an important strategy for viral anti-RNAi. In a number of studies, VSRs (TCV CP, CMV 2b, tombusvirus P19, PVX P25, polerovirus P0, and P1 of sweet potato mild mottle virus) have been identified that could target AGO1 for its degradation or interfering its function (Zhang et al., 2006; Bortolamiol et al., 2007; Azevedo et al., 2010; Chiu et al., 2010; Giner et al., 2010; Várallyay et al., 2010; Derrien et al., 2012). Except for AGO1, RNAi pathway key proteins of DCLs and other AGOs were also reported to be attacked by VSRs for disturbing their function (Lacombe et al., 2010; Hamera et al., 2012; Ramesh et al., 2014; Csorba et al., 2015). As an antivirus pathway initiated by viral-derived siRNAs, RNAi also defends against DNA viruses (Bisaro, 2006). It was reported that DICER-like 3 (DCL3) plays an important role in resistance against DNA viruses and presumably via DNA methylation (Akbergenov et al., 2006; Blevins et al., 2006; Raja et al., 2014). Several DNA virus-encoded VSRs have been identified to counteract RNAi including ACMV AC4 and AC2, CaLCuV AL2/AC2, TYLCCNV βC1, etc., (Chellappan et al., 2005; Cui et al., 2005; Buchmann et al., 2009). Except for directly targeting the RNAi pathway of virus VSRs, it has been reported recently that virus-encoded protein (CLCuMuV V2) could interfere with the interaction of CaM-CAMTA3 in calcium signaling, which positively regulates the gene transcription of RNAi key components RNA-dependent RNA polymerase 6 (RDR6) and Bifunctional nuclease-2 (BN2) to suppress RNAi (Wang et al., 2021b). These defense and counterdefense arm race between the plant’s defense machinery and viral VSRs are results of long co-evolutionary history and display a complex and sophisticated network.
Research progress about light-mediated RNAi signaling pathways is rare compared with those regulated by the phytohormone pathway. Most of these few articles focus on describing the effect of light intensity on the RNAi pathway (Kotakis et al., 2010, 2011; Patil and Fauquet, 2015). Some studies documented “high-light (HL)” intensity (130 ± 20 μmol m–2 s–1) positively affects the frequency of spontaneous post-transcriptional gene silencing (PTGS) in transgenic plants than “low-light (LL)” intensity (35 ± 15 μmol m–2 s–1) conditions (Kotakis et al., 2010). Furthermore, HL activates higher expression levels of DCL3 and DCL4 than that of LL, which further emphasized the regulation of light on RNAi (Kotakis et al., 2011). However, there is also some inconsistent report indicating not all the HL intensity is always good for promoting plant RNAi response. A too HL intensity (≥450 μE/m2/s) even confers a negative impact on the systemic movement of the silencing signal in transient agroinfiltration studies in N. benthamiana, whereas the viral symptom severity was reduced in this context. This phenomenon could be explained by a change in the plant sink-source relationship, which finally affected the systemic translocation of either small RNAs or the viral genome via the phloem (Patil and Fauquet, 2015).
The detailed mechanism of how light regulates the RNAi defense pathway is still elusive. Several future study areas could be explored to answer this important scientific question including what intensity or which spectrum of light can maximize the activation of plant RNAi pathways and what are the host factor(s) and mechanism(s) of light-regulated RNAi pathways?
Light-Mediated Protein Stability and Defense Responses
The protein stability regulation plays a central role in plant defensive responses against the invasion of pathogens including bacteria, fungi, and viruses. The 26S proteasome pathway commonly indicates the ubiquitin 26S proteasome degradation system (UPS) in which the degraded protein needs to be ubiquitinated by a series of ubiquitin-related enzymes, namely, ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase enzyme (E3) (Dielen et al., 2010). The polyubiquitinated target proteins were then loaded into 26S proteasome for their degradation. Plenty of articles have shown that the plants deploy UPS for disease resistance. Likewise, proteins encoded by several begomoviruses are direct targets of UPS degradation system. Begomovirus is the biggest plant virus genus consisting of more than 320 species and infects dicotyledonous plants. Most of them are the most destructive plant viral pathogens. Tomato yellow leaf curl virus (TYLCV), the coat protein (CP) encoded by its genome, is a target of UPS digestion (Gorovits et al., 2014). In addition, a well-known pathogenicity determinant βC1 encoded by TYLCCNV-associated betasatellite is found to be degraded by UPS as well (Shen et al., 2016). Besides viruses, other types of pathogens such as bacteria and fungi and their major pathogenic factors are also targeted by UPS. A recent study revealed a new ubiquitin-independent protein degradation pathway deployed by insect-vectored plant pathogenic phytoplasmas. This study demonstrated that SAP05 protein effectors from phytoplasmas hijacked the plant ubiquitin receptor RPN10 in a way that is independent of substrate ubiquitination, then promoted the concurrent degradation of two plant TFs, namely, SPL and GATA. This bacterial hijack of plant developmental regulators prolonged the host lifespan and induced witches’ broom-like proliferations of leaf and sterile shoots and ultimately facilitated parasitism of phytoplasma (Huang et al., 2021).
Autophagy has long been known as a conserved vacuole-/lysosome-mediated degradation pathway for clearing and recycling cellular components. Growing evidence has linked autophagy to immunity against invading pathogens, which elucidates the disease resistance roles of autophagy in plants. Autophagy-mediated plant defense responses are largely reported to be resistant to insect-borne viruses (Hofius et al., 2017; Ismayil et al., 2020; Yang et al., 2020). For instance, the key autophagy protein encoded by autophagy-related gene 8 (ATG8), which was reported to interact with βC1 encoded by CLCuMuV-associated betasatellite for degradation. Silencing of other essential proteins in the autophagy pathway, ATG5 and ATG7, reduced the resistance of the plant to a large number of DNA viruses, such as CLCuMuV, TYLCV, and TYLCCNV, which elucidated the significant roles of autophagy in plant defense (Haxim et al., 2017).
Studies about light signaling-mediating 26S proteasome and autophagy pathway to fight against attackers are rarely reported. Several well-known regulators functioning in promoting protein degradation in light signaling could provide examples for our understanding of this layer of light regulation on disease resistance. For example, phytochromes, such as phyA and phyB, mediate the degradation of PIFs via the 26S proteasome pathway for light response regulation (Park et al., 2018). Otherwise, the positive and negative factors of photomorphogenesis, PIF1 and HFR1, undergo reciprocal co-degradation via the 26S proteasome pathway in the dark to optimize photomorphogenesis (Xu et al., 2017). These studies illustrated the important roles of core factors in light signaling in protein accumulation regulation, which is possibly also essential for regulating the protein functions in other plant defense pathways. Actually, there are several vital regulators in plant resistance pathways, which are reported to be sensitive to light, such as ETHYLENE RESPONSE FACTOR1 (ERF1), a crucial factor in the biotic and abiotic stress responses, were reported to be unstable in the dark (Cheng et al., 2017). Other key factors of JA defense response pathway, MYCs and JAZs, their protein accumulations were also regulated by different light or dark conditions via 26S proteasome, and these processes were phytochromes dependent (Chico et al., 2014). These studies indicated the significant roles of light signaling in regulating protein degradation. Meanwhile, whether light signaling regulators could interact with proteins in the 26S proteasome or autophagy pathway, such as RPNs and autophagy-related proteins (ATGs), they wait for further exploring for understanding the detailed defensive response mechanisms regulated by light.
Light-Engineering Technology for Insect Vector Controlling
A key player in vector-borne pathogen transmission is the insect vectors, which facilitate pathogens to transmit among hosts. Therefore, inhibiting the population of vector insects or reducing their fitness on plants plays an important role in the prevention and control of vector-borne diseases. Due to the destructive effects of pesticides on the environment, biological and physical strategies for insect control will be very helpful for sustainable agriculture. Rapidly developed greenhouse agriculture has tried to supplement LED lighting systems to optimize crop production and quality, which provides a breakthrough and profound prospect for pest and disease controlling in a light-engineering technology (Gallé et al., 2021; Lazzarin et al., 2021). Therefore, it makes great sense for exploring the effects and mechanisms of light-mediated resistance to fight against herbivores. The effects of light on herbivores’ behavior consist of direct and indirect regulation. As the insects’ compound eyes contain visual pigments, which enable them to perceive different wavelengths of light. Therefore, revealing the mechanism by which light affects insect behaviors makes great sense for developing environmentally friendly insect control strategies. A recent study has elucidated the clock genes, temperature, and light affecting mosquito mating, it might lead to novel vector control strategies based on the light treatment that target insect reproductive behavior (Wang et al., 2021c). Similar to this, plant insect pests also have a visual system and it was reported that whiteflies were more attracted by a 550-nm wavelength of green LED and a 469-nm wavelength of the blue LED was proved to be most inhibitory (Stukenberg and Poehling, 2019). However, it might be more complicated when considering pest control through light in agriculture, as the indirect influence of light-mediated plant resistance to insect pests plays an important role. Therefore, uncovering the mechanisms of light signaling-regulated plant defense to herbivores has great significance in greenhouse agriculture. In the following part, we will review the indirect regulation of light on herbivores, which focuses on targeting different plant resistance pathways.
A number of studies have documented a series of the specific spectrum of light that could induce various plant defense responses to herbivores by distinguished mechanisms. For instance, UVB light enhances the resistance of multiple plant species, such as Arabidopsis, tobacco, rice, and maize to Spodoptera litura. The mechanism is through eliciting the JA-regulated glucosinolates (GSs) and an unidentified anti-insect metabolite(s) (Qi et al., 2018). Kegge et al. (2013) showed that low R:FR ratio and severe shading conditions suppressed both the constitutive and methyl jasmonate-induced volatiles and terpenoids in Arabidopsis, and volatile organic compound (VOC)-based preference of Pieris brassicae caterpillars was significantly affected by the R:FR ratio. Despite influencing anti-insect metabolites and volatiles of plants, other studies, which elucidated a distinguished mechanism of light-regulated plant defense to insects, are based on changing plant morphology. For example, cucumber seedlings treated by fluorescent lamps (FLs) (R:FR ratio was 7.0) were less attractive to whitefly than that of metal-halide lamps (MLs) (R:FR ratio was 1.2), which were considered probably due to changes in morphologic characteristics such as the leaf color and thickness resulting from high R:FR illumination of FL (Shibuya et al., 2010). Furthermore, phyB1phyB2 double mutant tomato showed reduced densities of glandular trichomes (Cortés et al., 2016). Escobar-Bravo et al. (2017) summarized the herbivore defense functions by UVB light treatment in different plants and insect interaction systems. Except for these light-regulated plants’ direct resistance to herbivores, a few studies demonstrated light-mediated plant indirect defense against herbivores, which was achieved by promoting host attractions to insect predators. For example, high R:FR ratio induced JA-controlled extrafloral nectar (ER) secretion of lima bean (Phaseolus lunatus). ER is considered to be activated by JA and functions as an indirect defense mechanism against herbivores (Radhika et al., 2010). Furthermore, inactivation of phyB regulated signaling, which mutated the two phyB genes in tomato or treated tomato plants with a low R:FR ratio compromised both the direct and indirect defenses, which induced by methyl jasmonate (MeJA). The result showed that predatory mirid bug (Macrolophus pygmaeus) preferred VOCs from plants in which phyB was inactivated over VOCs from the control plants (Cortés et al., 2016). All these studies elucidated the significant roles of light pathway in regulating plant resistance to herbivores.
Prospects of Light-Engineering Technology for Enhanced Plant Disease Resistance
The outspreading of tremendous emergence and reemergence of plant diseases are mostly caused by vector insects, which accelerate the transmission of pathogens among hosts (Gao et al., 2010; Heck and Brault, 2018; Wang et al., 2019; Chiapello et al., 2021). Therefore, researching for efficient strategies to prevent and control insect-borne diseases is of great significance for ensuring the safety of food production. The mechanism of light-mediated plant resistance will provide new directions for developing plant disease control strategies based on light treatment and crop breeding. From the studies reported previously, it could be concluded that most studies illustrated red light as a positive regulator for plant defense response and FR light as a negative factor. Most of the literature has demonstrated the impaired resistance of plants to pathogens under FR light treatment (Cerrudo et al., 2012; de Wit et al., 2013; Courbier et al., 2020, 2021). That explains why the red spectrum of LED lighting systems is usually used in greenhouses for crop disease management (Gallé et al., 2021; Lazzarin et al., 2021). According to the resistant functions of different spectrums of light to distinguish pathogens and herbivores, we can design optimized combinations of specific wavelength and intensity of light to prevent and control specific types of plant diseases. Based on the studies on the host resistance responses of different light treatments to cure various plant diseases, the environmental light quality and intensity could be tailed for light engineering with LEDs in an individual greenhouse for precise disease control. Of course, with the assistance of artificial intelligence technology, it is also possible to balance crop yield and disease resistance by light-engineering technology. We can setup an intelligent light control system to satisfy different requirements of plants (disease resistance or growth), which provides the optimal growth conditions at each growth stage of the plants. Furthermore, we can also use different lights to prevent and control different plant diseases. These ideas about disease prevention and control in green agriculture are all based on the mechanism revelation of plant defense to different pathogens or herbivores by specific light treatment.
Besides understanding specific wavelength of light-mediated plant resistance to pathogens, the exploration of molecular mechanisms is also pivotal when considering applied purposes in breeding disease-resistant crops. Other studies could also explore the mechanisms of how light signaling regulates plant resistance. For example, which photoreceptor(s) regulate the biosynthesis of terpenoid and other VOCs, which function as communication signals with the host plant community together with various insects, e.g., vector, non-vector, predator, and parasitoids? How does light signaling intercross with the RNAi pathway? The rapid development of gene editing technology can enable us to obtain crops with both disease resistance and high-yielding traits.
Conclusion
Exploring novel resistance pathways in plants is of great significance for enhancing the broad-spectrum disease resistance of crops. In this review, we propose a new idea about light-engineering technology combining both the optimal external LED-based environmental conditions and optimal plant internal disease gene networks. The final aim is to provide enhanced disease resistance and high yield crop performance. Tailed light conditions confer better plant resistance against insect-borne pathogens and insect vectors. Exploring how to use light to control insect-borne diseases will provide broad prospects for the development of green agriculture. We believe that light-engineering technology, which is combined with big data technology and LED technology, will provide human beings with a high yield of crops and also safer food in the future.
Author Contributions
JY and DW conceived this manuscript. DW performed the literature search and drafted the manuscript. JY, JQ, and BD revised the manuscript. All authors contributed to the article and approved the submitted version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program (2019YFC1200503 and 2021YFD1400800) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDPB16). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul Malik, N. A., Kumar, I. S., and Nadarajah, K. (2020). Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 21, 963. doi: 10.3390/ijms21030963
Ahn, S. Y., Kim, S. A., and Yun, H. K. (2015). Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. Eur. J. Plant Pathol. 143, 753–765. doi: 10.1007/s10658-015-0725-5
Akbergenov, R., Si-Ammour, A., Blevins, T., Amin, I., Kutter, C., Vanderschuren, H., et al. (2006). Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucleic Acids Res. 34, 462–471. doi: 10.1093/nar/gkj447
Al-Sady, B., Ni, W., Kircher, S., Schäfer, E., and Quail, P. H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell. 23, 439–446. doi: 10.1016/j.molcel.2006.06.011
Azevedo, J., Garcia, D., Pontier, D., Ohnesorge, S., Yu, A., Garcia, S., et al. (2010). Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev. 24, 904–915. doi: 10.1101/gad.19087
Balint-Kurti, P. (2019). The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol. 20, 1163–1178. doi: 10.1111/mpp.12821
Bigeard, J., Colcombet, J., and Hirt, H. (2015). Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant. 8, 521–539. doi: 10.1016/j.molp.2014.12.022
Bisaro, D. M. (2006). Silencing suppression by geminivirus proteins. Virology 344, 158–168. doi: 10.1016/j.virol.2005.09.041
Blevins, T., Rajeswaran, R., Shivaprasad, P. V., Beknazariants, D., Si-Ammour, A., Park, H. S., et al. (2006). Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 34, 6233–6246. doi: 10.1093/nar/gkl886
Bortolamiol, D., Pazhouhandeh, M., Marrocco, K., Genschik, P., and Ziegler-Graff, V. (2007). The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol. 17, 1615–1621. doi: 10.1016/j.cub.2007.07.061
Boutrot, F., and Zipfel, C. (2017). Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol 55, 257–286. doi: 10.1146/annurev-phyto-080614-120106
Buchmann, R. C., Asad, S., Wolf, J. N., Mohannath, G., and Bisaro, D. M. (2009). Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J. Virol. 83, 5005–5013. doi: 10.1128/JVI.01771-08
Cargnel, M. D., Demkura, P. V., and Ballaré, C. L. (2014). Linking phytochrome to plant immunity: low red: far-red ratios increase Arabidopsis susceptibility to Botrytis cinerea by reducing the biosynthesis of indolic glucosinolates and camalexin. New Phytol. 204, 342–354. doi: 10.1111/nph.13032
Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427. doi: 10.1146/annurev-arplant-050312-120221
Cerrudo, I., Keller, M. M., Cargnel, M. D., Demkura, P. V., de Wit, M., Patitucci, M. S., et al. (2012). Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 158, 2042–2052. doi: 10.1104/pp.112.193359
Chandra-Shekara, A. C., Gupte, M., Navarre, D., Raina, S., Raina, R., Klessig, D., et al. (2006). Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J. 45, 320–334. doi: 10.1111/j.1365-313X.2005.02618.x
Chellappan, P., Vanitharani, R., and Fauquet, C. M. (2005). MicroRNA-binding viral protein interferes with Arabidopsis development. Proc. Natl. Acad. Sci. U.S.A. 102, 10381–10386. doi: 10.1073/pnas.0504439102
Chen, H., Köllner, T. G., Li, G., Wei, G., Chen, X., Zeng, D., et al. (2020). Combinatorial evolution of a terpene synthase gene cluster explains terpene variations in Oryza. Plant Physiol. 182, 480–492. doi: 10.1104/pp.19.00948
Chen, L. J., Zhao, F. F., Zhang, M., Lin, H. H., and Xi, D. H. (2015). Effects of light quality on the interaction between cucumber mosaic virus and nicotiana tabacum. J. Phytopathol. 163, 1002–1013. doi: 10.1111/jph.12408
Cheng, M. C., Kuo, W. C., Wang, Y. M., Chen, H. Y., and Lin, T. P. (2017). UBC18 mediates ERF1 degradation under light-dark cycles. New Phytol. 213, 1156–1167. doi: 10.1111/nph.14272
Chiapello, M., Bosco, L., Ciuffo, M., Ottati, S., Salem, N., Rosa, C., et al. (2021). Complexity and Local Specificity of the Virome Associated with Tospovirus-Transmitting Thrips Species. J. Virol. 95, e0059721. doi: 10.1128/JVI.00597-21
Chico, J.-M., Fernández-Barbero, G., Chini, A., Fernández-Calvo, P., Díez-Díaz, M., and Solano, R. (2014). Repression of jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in arabidopsis. Plant Cell 26, 1967–1980. doi: 10.1105/tpc.114.125047
Chiu, M. H., Chen, I. H., Baulcombe, D. C., and Tsai, C. H. (2010). The silencing suppressor P25 of potato virus X interacts with argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 11, 641–649. doi: 10.1111/j.1364-3703.2010.00634.x
Christie, J. M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45. doi: 10.1146/annurev.arplant.58.032806.103951
Cloix, C., Kaiserli, E., Heilmann, M., Baxter, K. J., Brown, B. A., O’Hara, A., et al. (2012). C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc. Natl. Acad. Sci. U.S.A. 109, 16366–16370. doi: 10.1073/pnas.1210898109
Correll, M. J., Coveney, K. M., Raines, S. V., Mullen, J. L., Hangarter, R. P., and Kiss, J. Z. (2003). Phytochromes play a role in phototropism and gravitropism in Arabidopsis roots. Adv. Space Res. 31, 2203–2210. doi: 10.1016/s0273-1177(03)00245-x
Cortés, L. E., Weldegergis, B. T., Boccalandro, H. E., Dicke, M., and Ballaré, C. L. (2016). Trading direct for indirect defense? Phytochrome B inactivation in tomato attenuates direct anti-herbivore defenses whilst enhancing volatile-mediated attraction of predators. New Phytol. 212, 1057–1071. doi: 10.1111/nph.142
Courbier, S., Grevink, S., Sluijs, E., Bonhomme, P.-O., Kajala, K., Van Wees, S. C. M., et al. (2020). Far-red light promotes Botrytis cinerea disease development in tomato leaves via jasmonate-dependent modulation of soluble sugars. Plant Cell Environ. 43, 2769–2781. doi: 10.1111/pce.13870
Courbier, S., Snoek, B. L., Kajala, K., Li, L., van Wees, S. C. M., and Pierik, R. (2021). Mechanisms of far-red light-mediated dampening of defense against Botrytis cinerea in tomato leaves. Plant Physiol. 187, 1250–1266. doi: 10.1093/plphys/kiab354
Csorba, T., Kontra, L., and Burgyán, J. (2015). Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 47, 85–103. doi: 10.1016/j.virol.2015.02.028
Cui, X., Li, G., Wang, D., Hu, D., and Zhou, X. (2005). A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J. Virol. 79, 10764–10775. doi: 10.1128/JVI.79.16.10764-10775.2005
Dáder, B., Legarrea, S., Moreno, A., Plaza, M., Carmo-Sousa, M., Amor, F., et al. (2015). Control of insect vectors and plant viruses in protected crops by novel pyrethroid-treated nets. Pest. Manag. Sci. 71, 1397–1406. doi: 10.1002/ps.3942
D’Amico-Damião, V., and Carvalho, R. F. (2018). Cryptochrome-Related Abiotic Stress Responses in Plants. Front. Plant Sci. 9:1897. doi: 10.3389/fpls.2018.01897
de Wit, M., Spoel, S. H., Sanchez-Perez, G. F., Gommers, C. M. M., Pieterse, C. M. J., Voesenek, L., et al. (2013). Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 75, 90–103. doi: 10.1111/tpj.12203
Demarsy, E., Schepens, I., Okajima, K., Hersch, M., Bergmann, S., Christie, J., et al. (2012). Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 31, 3457–3467. doi: 10.1038/emboj.2012.186
Demkura, P. V., and Ballaré, C. L. (2012). UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol. Plant 5, 642–652. doi: 10.1093/mp/sss025
Derrien, B., Baumberger, N., Schepetilnikov, M., Viotti, C., De Cillia, J., Ziegler-Graff, V., et al. (2012). Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. U.S.A. 109, 15942–15946. doi: 10.1073/pnas.1209487109
Dielen, A.-S., Badaoui, S., Candresse, T., and German-Retana, S. (2010). The ubiquitin/26S proteasome system in plant-pathogen interactions: a never-ending hide-and-seek game. Mol. Plant Pathol. 11, 293–308. doi: 10.1111/j.1364-3703.2009.00596.x
Ding, Y., Sun, T., Ao, K., Peng, Y., Zhang, Y., Li, X., et al. (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454. doi: 10.1016/j.cell.2018.03.044
Ðinh, S. T., Gális, I., and Baldwin, I. T. (2013). UVB radiation and 17-hydroxygeranyllinalool diterpene glycosides provide durable resistance against mirid (Tupiocoris notatus) attack in field-grown Nicotiana attenuata plants. Plant Cell Environ. 36, 590–606. doi: 10.1111/j.1365-3040.2012.02598.x
Erickson, F. L., Dinesh-Kumar, S. P., Holzberg, S., Ustach, C. V., Dutton, M., Handley, V., et al. (1999). Interactions between tobacco mosaic virus and the tobacco N gene. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 653–658. doi: 10.1098/rstb.1999.0417
Escobar-Bravo, R., Chen, G., Kim, H. K., Grosser, K., van Dam, N. M., Leiss, K. A., et al. (2019). Ultraviolet radiation exposure time and intensity modulate tomato resistance to herbivory through activation of jasmonic acid signaling. J. Exp. Bot. 70, 315–327. doi: 10.1093/jxb/ery347
Escobar-Bravo, R., Klinkhamer, P. G. L., and Leiss, K. A. (2017). Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against Arthropod Herbivores. Front. Plant Sci. 8:278. doi: 10.3389/fpls.2017.00278
Fernández-Milmanda, G. L., and Ballaré, C. L. (2021). Shade avoidance: expanding the color and hormone palette. Trends Plant Sci. 26, 509–523. doi: 10.1016/j.tplants.2020.12.006
Fernández-Milmanda, G. L., Crocco, C. D., Reichelt, M., Mazza, C. A., Köllner, T. G., Zhang, T., et al. (2020). A light-dependent molecular link between competition cues and defence responses in plants. Nat. Plants 6, 223–230. doi: 10.1038/s41477-020-0604-8
Ferrarezi, R. S., Vincent, C. I., Urbaneja, A., and Machado, M. A. (2020). Editorial: unravelling citrus huanglongbing disease. Front. plant sci. 11:609655–609655. doi: 10.3389/fpls.2020.609655
Folta, K. M., and Maruhnich, S. A. (2007). Green light: a signal to slow down or stop. J. Exp. Bot. 58, 3099–3111. doi: 10.1093/jxb/erm130
Gallé, Á, Czékus, Z., Tóth, L., Galgóczy, L., and Poór, P. (2021). Pest and disease management by red light. Plant Cell Environ 10, 3197–3210. doi: 10.1111/pce.14142
Gao, S., Qu, J., Chua, N.-H., and Ye, J. (2010). A new strain of Indian cassava mosaic virus causes a mosaic disease in the biodiesel crop Jatropha curcas. Arch. Virol. 155, 607–612. doi: 10.1007/s00705-010-0625-0
Giner, A., Lakatos, L., García-Chapa, M., López-Moya, J. J., and Burgyán, J. (2010). Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog 6:e1000996. doi: 10.1371/journal.ppat.1000996
Gorovits, R., Moshe, A., Ghanim, M., and Czosnek, H. (2014). Degradation mechanisms of the Tomato yellow leaf curl virus coat protein following inoculation of tomato plants by the whitefly Bemisia tabaci. Pest. Manag. Sci. 70, 1632–1639. doi: 10.1002/ps.3737
Hamera, S., Song, X., Su, L., Chen, X., and Fang, R. (2012). Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant J. 69, 104–115. doi: 10.1111/j.1365-313X.2011.04774.x
Haxim, Y., Ismayil, A., Jia, Q., Wang, Y., Zheng, X., Chen, T., et al. (2017). Autophagy functions as an antiviral mechanism against geminiviruses in plants. eLife 6, e23897. doi: 10.7554/eLife.23897
Heck, M., and Brault, V. (2018). Targeted disruption of aphid transmission: a vision for the management of crop diseases caused by Luteoviridae members. Curr. Opin. Virol. 33, 24–32. doi: 10.1016/j.coviro.2018.07.007
Heijde, M., and Ulm, R. (2012). UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 17, 230–237. doi: 10.1016/j.tplants.2012.01.007
Hoang, Q. T. N., Han, Y. J., and Kim, J. I. (2019). Plant Phytochromes and their Phosphorylation. Int. J. Mol. Sci. 20, 3450. doi: 10.3390/ijms20143450
Hofius, D., Li, L., Hafrén, A., and Coll, N. S. (2017). Autophagy as an emerging arena for plant–pathogen interactions. Curr. Opin. Plant Biol. 38, 117–123. doi: 10.1016/j.pbi.2017.04.017
Huang, W., MacLean, A. M., Sugio, A., Maqbool, A., Busscher, M., Cho, S. T., et al. (2021). Parasitic modulation of host development by ubiquitin-independent protein degradation. Cell 184, 5201. doi: 10.1016/j.cell.2021.08.029
Huang, X., Ouyang, X., Yang, P., Lau, O. S., Chen, L., Wei, N., et al. (2013). Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc. Natl. Acad. Sci. U.S.A. 110, 16669–16674. doi: 10.1073/pnas.13166221
Ismayil, A., Yang, M., and Liu, Y. (2020). Role of autophagy during plant-virus interactions. Semin. Cell Dev. Biol. 101, 36–40. doi: 10.1016/j.semcdb.2019.07.001
Izaguirre, M. M., Mazza, C. A., Biondini, M., Baldwin, I. T., and Ballaré, C. L. (2006). Remote sensing of future competitors: impacts on plant defenses. Proc. Natl. Acad. Sci. U.S.A. 103, 7170–7174. doi: 10.1073/pnas.0509805103
Jeong, R. D., Kachroo, A., and Kachroo, P. (2010). Blue light photoreceptors are required for the stability and function of a resistance protein mediating viral defense in Arabidopsis. Plant Signal. Behav. 5, 1504–1509. doi: 10.4161/psb.5.11.13705
Jiao, Y., Lau, O. S., and Deng, X. W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8, 217–230. doi: 10.1038/nrg2049
Ju, C., and Chang, C. (2015). Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 169, 85–95. doi: 10.1104/pp.15.00845
Kangasjärvi, S., Tikkanen, M., Durian, G., and Aro, E. M. (2014). Photosynthetic light reactions–an adjustable hub in basic production and plant immunity signaling. Plant Physiol. Biochem. 81, 128–134. doi: 10.1016/j.plaphy
Kazan, K., and Manners, J. M. (2011). The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 62, 4087–4100. doi: 10.1093/jxb/err142
Kegge, W., Weldegergis, B. T., Soler, R., Eijk, M. V., Dicke, M., Voesenek, L., et al. (2013). Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol. 200, 861–874. doi: 10.1111/nph.12407
Kotakis, C., Vrettos, N., Daskalaki, M. G., Kotzabasis, K., and Kalantidis, K. (2011). DCL3 and DCL4 are likely involved in the light intensity-RNA silencing cross talk in Nicotiana benthamiana. Plant Signal. Behav. 6, 1180–1182. doi: 10.4161/psb.6.8.15689
Kotakis, C., Vrettos, N., Kotsis, D., Tsagris, M., Kotzabasis, K., and Kalantidis, K. (2010). Light intensity affects RNA silencing of a transgene in Nicotiana benthamiana plants. BMC Plant Biol 10, 220. doi: 10.1186/1471-2229-10-220
Lacombe, S., Bangratz, M., Vignols, F., and Brugidou, C. (2010). The rice yellow mottle virus P1 protein exhibits dual functions to suppress and activate gene silencing. Plant J. 61, 371–382. doi: 10.1111/j.1365-313X.2009.04062.x
Lakatos, L., Szittya, G., Silhavy, D., and Burgyán, J. (2004). Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J. 23, 876–884. doi: 10.1038/sj.emboj.7600096
Lau, O. S., and Deng, X. W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17, 584–593. doi: 10.1016/j.tplants.2012.05.004
Lazebnik, J., Frago, E., Dicke, M., and van Loon, J. J. A. (2014). Phytohormone mediation of interactions between herbivores and plant pathogens. J. Chem. Ecol. 40, 730–741. doi: 10.1007/s10886-014-0480-7
Lazzarin, M., Meisenburg, M., Meijer, D., van Ieperen, W., Marcelis, L. F. M., Kappers, I. F., et al. (2021). LEDs Make it resilient: effects on plant growth and defense. Trends Plant Sci. 26, 496–508. doi: 10.1016/j.tplants.2020.11.013
Leone, M., Keller, M. M., Cerrudo, I., and Ballaré, C. L. (2014). To grow or defend? Low red: far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. New Phytol. 204, 355–367. doi: 10.1111/nph.12971
Li, F., and Ding, S.-W. (2006). Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu. Rev. Microbiol. 60, 503–531. doi: 10.1146/annurev.micro.60.080805.142205
Li, J., Terzaghi, W., Gong, Y., Li, C., Ling, J. J., Fan, Y., et al. (2020). Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat. Commun. 11, 1592. doi: 10.1038/s41467-020-15394-7
Li, N., Han, X., Feng, D., Yuan, D., and Huang, L. J. (2019). Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do We understand what they are whispering? Int. J. Mol. Sci. 20, 671. doi: 10.3390/ijms20030671
Li, R., Weldegergis, B. T., Li, J., Jung, C., Qu, J., Sun, Y., et al. (2014). Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 26, 4991–5008. doi: 10.1105/tpc.114.133181
Li, Y., Cheah, B. H., Fang, Y. F., Kuang, Y. H., Lin, S. C., Liao, C. T., et al. (2021). Transcriptomics identifies key defense mechanisms in rice resistant to both leaf-feeding and phloem feeding herbivores. BMC Plant Biol. 21:306. doi: 10.1186/s12870-021-03068-5
Liang, T., Mei, S., Shi, C., Yang, Y., Peng, Y., Ma, L., et al. (2018). UVR8 Interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis. Dev. Cell 44, 512. doi: 10.1016/j.devcel.2017.12.028
Liang, T., Yang, Y., and Liu, H. (2019). Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol. 221, 1247–1252. doi: 10.1111/nph.15469
Liu, B., Yang, Z., Gomez, A., Liu, B., Lin, C., and Oka, Y. (2016). Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J. Plant Res. 129, 137–148. doi: 10.1007/s10265-015-0782-z
Liu, H., Liu, B., Zhao, C., Pepper, M., and Lin, C. (2011). The action mechanisms of plant cryptochromes. Trends Plant Sci. 16, 684–691. doi: 10.1016/j.tplants.2011.09.002
Liu, M., Zhang, S., Hu, J., Sun, W., Padilla, J., He, Y., et al. (2019). Phosphorylation-guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proc. Natl. Acad. Sci. U.S.A. 116, 17572–17577. doi: 10.1073/pnas.1905123116
Liu, X., Rockett, K. S., Kørner, C. J., and Pajerowska-Mukhtar, K. M. (2015). Salicylic acid signalling: new insights and prospects at a quarter-century milestone. Essays Biochem. 58, 101–113. doi: 10.1042/bse0580101
Lu, J., Ju, H., Zhou, G., Zhu, C., Erb, M., Wang, X., et al. (2011). An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 68, 583–596. doi: 10.1111/j.1365-313X.2011.04709.x
Mallory, A. C., Reinhart, B. J., Bartel, D., Vance, V. B., and Bowman, L. H. (2002). A viral suppressor of RNA silencing differentially regulates the accumulation of short interfering RNAs and micro-RNAs in tobacco. Proc. Natl. Acad. Sci. U.S.A. 99, 15228–15233. doi: 10.1073/pnas.232434999
Martínez, de Alba, A. E., Elvira-Matelot, E., and Vaucheret, H. (2013). Gene silencing in plants: a diversity of pathways. Biochim Biophys Acta 1829, 1300–1308. doi: 10.1016/j.bbagrm.2013
Montes, N., and Pagán, I. (2019). Light intensity modulates the efficiency of virus seed transmission through modifications of plant Tolerance. Plants (Basel) 8, 304. doi: 10.3390/plants8090304
Muhammad, T., Zhang, F., Zhang, Y., and Liang, Y. (2019). RNA Interference: a natural immune system of plants to counteract biotic stressors. Cells 8, 38. doi: 10.3390/cells8010038
Nakahara, K. S., and Masuta, C. (2014). Interaction between viral RNA silencing suppressors and host factors in plant immunity. Curr. Opin. Plant Biol. 20, 88–95. doi: 10.1016/j.pbi.2014.05.004
Ngou, B. P. M., Ahn, H. K., Ding, P., and Jones, J. D. G. (2021). Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115. doi: 10.1038/s41586-021-03315-7
Niehl, A., and Heinlein, M. (2019). Perception of double-stranded RNA in plant antiviral immunity. Mol. Plant Pathol. 20, 1203–1210. doi: 10.1111/mpp.12798
Onkokesung, N., Reichelt, M., van Doorn, A., Schuurink, R. C., and Dicke, M. (2016). Differential costs of two distinct resistance mechanisms induced by different herbivore species in Arabidopsis. Plant Physiol. 170, 891–906. doi: 10.1104/pp.15.01780
Paik, I., and Huq, E. (2019). Plant photoreceptors: multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 92, 114–121. doi: 10.1016/j.semcdb.2019.03.007
Park, E., Kim, J., Lee, Y., Shin, J., Oh, E., Chung, W. I., et al. (2004). Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45, 968–975. doi: 10.1093/pcp/pch125
Park, E., Kim, Y., and Choi, G. (2018). Phytochrome B requires PIF degradation and sequestration to induce light responses across a wide range of light conditions. Plant Cell 30, 1277–1292. doi: 10.1105/tpc.17.00913
Patil, B. L., and Fauquet, C. M. (2015). Light intensity and temperature affect systemic spread of silencing signal in transient agroinfiltration studies. Mol. Plant Pathol. 16, 484–494. doi: 10.1111/mpp.12205
Pierik, R., and de Wit, M. (2014). Shade avoidance: phytochrome signalling and other aboveground neighbour detection cues. J. Exp. Bot. 65, 2815–2824. doi: 10.1093/jxb/ert389
Qi, J., Zhang, M., Lu, C., Hettenhausen, C., Tan, Q., Cao, G., et al. (2018). Ultraviolet-B enhances the resistance of multiple plant species to lepidopteran insect herbivory through the jasmonic acid pathway. Sci Rep 8, 277. doi: 10.1038/s41598-017-18600-7
Radhika, V., Kost, C., Mithöfer, A., and Boland, W. (2010). Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proc. Natl. Acad. Sci. U.S.A. 107, 17228–17233. doi: 10.1073/pnas.1009007107
Raja, P., Jackel, J. N., Li, S., Heard, I. M., and Bisaro, D. M. (2014). Arabidopsis double-stranded RNA binding protein DRB3 participates in methylation-mediated defense against geminiviruses. J. Virol. 88, 2611–2622. doi: 10.1128/JVI.02305-13
Ramesh, S. V., Ratnaparkhe, M. B., Kumawat, G., Gupta, G. K., and Husain, S. M. (2014). Plant mirnaome and antiviral resistance: a retrospective view and prospective challenges. Virus Genes 48, 1–14. doi: 10.1007/s11262-014-1038-z
Roeber, V. M., Bajaj, I., Rohde, M., Schmülling, T., and Cortleven, A. (2021). Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645–664. doi: 10.1111/pce.13948
Roig-Villanova, I., Paulišić, S., and Martinez-Garcia, J. F. (2019). Shade avoidance and neighbor detection. Methods Mol. Biol. 2026, 157–168. doi: 10.1007/978-1-4939-9612-4_13
Ruan, J., Zhou, Y., Zhou, M., Yan, J., Khurshid, M., Weng, W., et al. (2019). Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 20, 2479. doi: 10.3390/ijms20102479
Saijo, Y., Loo, E. P.-I., and Yasuda, S. (2018). Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 93, 592–613. doi: 10.1111/tpj.13808
Schweizer, F., Fernández-Calvo, P., Zander, M., Diez-Diaz, M., Fonseca, S., Glauser, G., et al. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25, 3117–3132. doi: 10.1105/tpc.113.115139
Shen, H., Moon, J., and Huq, E. (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 44, 1023–1035. doi: 10.1111/j.1365-313X.2005.02606.x
Shen, H., Zhu, L., Castillon, A., Majee, M., Downie, B., and Huq, E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20, 1586–1602. doi: 10.1105/tpc.108.060020
Shen, Q., Hu, T., Bao, M., Cao, L., Zhang, H., Song, F., et al. (2016). Tobacco RING E3 ligase NtRFP1 mediates ubiquitination and proteasomal degradation of a geminivirus-encoded βC1. Mol. Plant 9, 911–925. doi: 10.1016/j.molp.2016.03.008
Shibuya, T., Komuro, J., Hirai, N., Sakamoto, Y., Endo, R., and Kitaya, Y. (2010). Preference of sweetpotato whitefly adults to cucumber seedlings grown under two different light sources. Horttechnology 20, 873–876. doi: 10.21273/HORTTECH.20.5.873
Song, L., Gao, S., Jiang, W., Chen, S., Liu, Y., Zhou, L., et al. (2011). Silencing suppressors: viral weapons for countering host cell defenses. Protein Cell 2, 273–281. doi: 10.1007/s13238-011-1037-y
Song, Y. H., Estrada, D. A., Johnson, R. S., Kim, S. K., Lee, S. Y., MacCoss, M. J., et al. (2014). Distinct roles of FKF1, Gigantea, and Zeitlupe proteins in the regulation of constans stability in Arabidopsis photoperiodic flowering. Proc. Natl. Acad. Sci. U.S.A. 111, 17672–17677. doi: 10.1073/pnas.1415375111
Stukenberg, N., and Poehling, H. M. (2019). Blue-green opponency and trichromatic vision in the greenhouse whitefly (Trialeurodes vaporariorum) explored using light emitting diodes. Ann. Appl. Biol. 175, 146–163. doi: 10.1111/aab.12524
Szalai, G., Majláth, I., Pál, M., Gondor, O. K., Rudnóy, S., Oláh, C., et al. (2018). Janus-Faced nature of light in the cold acclimation processes of maize. Front. Plant Sci. 19:9–850. doi: 10.3389/fpls.2018.00850
Tholl, D. (2015). Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 148, 63–106. doi: 10.1007/10_2014_295
Ueno, M., Imaoka, A., Kihara, J., and Arase, S. (2007). Effects of light quality on induction of tryptamine-mediated resistance in lesion mimic mutant of rice infected with Magnaporthe grisea. J. Phytopathol. 155, 228–235. doi: 10.1111/j.1439-0434.2007.01222.x
Várallyay, E., Válóczi, A., Agyi, A., Burgyán, J., and Havelda, Z. (2010). Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J. 29, 3507–3519. doi: 10.1038/emboj.20
Voinnet, O., Lederer, C., and Baulcombe, D. C. (2000). A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103, 157–167.
Waadt, R. (2020). Phytohormone signaling mechanisms and genetic methods for their modulation and detection. Curr. Opin. Plant Biol. 57, 31–40. doi: 10.1016/j.pbi.2020.05.011
Waheed, S., Anwar, M., Saleem, M. A., Wu, J., Tayyab, M., and Hu, Z. (2021). The Critical Role of Small RNAs in Regulating Plant Innate Immunity. Biomolecules 11, 184. doi: 10.3390/biom11020184
Wang, D., Yao, X., Huang, G., Shi, T., Wang, G., and Ye, J. (2019). First report of Sri Lankan cassava mosaic virus infected cassava in China. Plant Dis. 103, 1437. doi: 10.1094/PDIS-09-20-1868-PDN
Wang, F., Wang, X., Zhang, Y., Yan, J., Ahammed, G. J., Bu, X., et al. (2021a). SlFHY3 and SlHY5 act compliantly to enhance cold tolerance through the integration of myo-inositol and light signaling in tomato. New Phytol. doi: 10.1111/nph.17934
Wang, Y., Gong, Q., Wu, Y., Huang, F., Ismayil, A., Zhang, D., et al. (2021b). A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe. 29, 1393. doi: 10.1016/j.chom.2021.07.003
Wang, G., Vega-Rodríguez, J., Diabate, A., Liu, J., Cui, C., Nignan, C., et al. (2021c). Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science 371, 411–415. doi: 10.1126/science.abd4359
Wang, H., Jiang, Y. P., Yu, H. J., Xia, X. J., Shi, K., Zhou, Y. H., et al. (2010). Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 127, 125–135. doi: 10.1007/s10658-009-9577-1
Wang, Q., and Lin, C. (2020). Mechanisms of cryptochrome-mediated photoresponses in plants. Annu. Rev. Plant Biol. 71, 103–129. doi: 10.1146/annurev-arplant-050718-100300
Wu, L., and Yang, H. Q. (2010). CRYPTOCHROME 1 Is Implicated in promoting R protein-mediated plant resistance to pseudomonas syringae in Arabidopsis. Mol. Plant 3, 539–548. doi: 10.1093/mp/ssp107
Wu, X., Xu, S., Zhao, P., Zhang, X., Yao, X., Sun, Y., et al. (2019). The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance. PLoS Pathog 15:e1007897. doi: 10.1371/journal.ppat.1007897
Wu, X., and Ye, J. (2020). Manipulation of Jasmonate Signaling by Plant Viruses and Their Insect Vectors. Viruses 12, 148. doi: 10.3390/v12020148
Xu, X., Kathare, P. K., Pham, V. N., Bu, Q., Nguyen, A., and Huq, E. (2017). Reciprocal proteasome-mediated degradation of PIFs and HFR1 underlies photomorphogenic development in Arabidopsis. Development 144, 1831–1840. doi: 10.1242/dev.146936
Yalpani, N., Enyedi, A. J., Leon, J., and Raskin, I. (1994). Ultraviolet-light and ozone stimulate accumulation of salicylic-acid, pathogenesis-related proteins and virus-resistance in tobacco. Planta 193, 372–376.
Yang, M., Ismayil, A., and Liu, Y. (2020). Autophagy in Plant-Virus Interactions. Annu. Rev. Virol. 7, 403–419. doi: 10.1146/annurev-virology-010220-054709
Yang, Y., Liang, T., Zhang, L., Shao, K., Gu, X., Shang, R., et al. (2018a). UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis. Nat. Plants 4, 98–107. doi: 10.1038/s41477-017-0099-0
Yang, Y. X., Wu, C. Q., Ahammed, G. J., Wu, C. J., Yang, Z. M., Wan, C. P., et al. (2018b). Red Light-induced systemic resistance against root-knot nematode is mediated by a coordinated regulation of salicylic acid, jasmonic acid and redox signaling in watermelon. Front. Plant Sci. 9:899. doi: 10.3389/fpls.2018.00899
Yang, Y. X., Wang, M. M., Yin, Y. L., Onac, E., Zhou, G. F., Peng, S., et al. (2015). RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genomics 16:120. doi: 10.1186/s12864-015-1228-7
Ye, J., Zhang, L., Zhang, X., Wu, X., and Fang, R. (2021). Plant Defense Networks against Insect-Borne Pathogens. Trends Plant Sci. 26, 272–287. doi: 10.1016/j.tplants.2020
Yuan, M., Jiang, Z., Bi, G., Nomura, K., Liu, M., Wang, Y., et al. (2021). Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109. doi: 10.1038/s41586-021-03316-6
Zavala, J. A., Mazza, C. A., Dillon, F. M., Chludil, H. D., and Ballaré, C. L. (2015). Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant Cell Environ. 38, 920–928. doi: 10.1111/pce.12368
Zhai, Y., Peng, H., Neff, M. M., and Pappu, H. R. (2020). Emerging Molecular Links Between Plant Photomorphogenesis and Virus Resistance. Front. Plant Sci. 11:920. doi: 10.3389/fpls.2020.00920
Zhang, L., Shang, J., Wang, W., Du, J., Li, K., Wu, X., et al. (2019). Comparison of transcriptome differences in soybean response to soybean mosaic virus under normal light and in the shade. Viruses 11, 793. doi: 10.3390/v11090793
Zhang, X., Yuan, Y. R., Pei, Y., Lin, S. S., Tuschl, T., Patel, D. J., et al. (2006). Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 20, 3255–3268. doi: 10.1101/gad.1495506
Zhang, Y., and Li, X. (2019). Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 50, 29–36. doi: 10.1016/j.pbi.2019.02.004
Zhao, P., Yao, X., Cai, C., Li, R., Du, J., Sun, Y., et al. (2019). Viruses mobilize plant immunity to deter nonvector insect herbivores. Sci. Adv. 5, eaav9801. doi: 10.1126/sciadv.aav9801
Zhao, P., Zhang, X., Gong, Y., Wang, D., Xu, D., Wang, N., et al. (2021). Red-light is an environmental effector for mutualism between begomovirus and its vector whitefly. PLoS Pathog 17e:1008770. doi: 10.1371/journal.ppat.1008770
Zhou, J. M., and Zhang, Y. (2020). Plant Immunity: danger perception and signaling. Cell 181, 978–989. doi: 10.1016/j.cell.2020.04.028
Zhu, M., van Grinsven, I. L., Kormelink, R., and Tao, X. (2019). Paving the way to tospovirus infection: multilined interplays with plant innate immunity. Annu. Rev. Phytopathol. 57, 41–62. doi: 10.1146/annurev-phyto-082718-100309
Zoltowski, B. D., and Imaizumi, T. (2014). Structure and Function of the ZTL/FKF1/LKP2 Group Proteins in Arabidopsis. Enzymes 35, 213–239. doi: 10.1016/B978-0-12-801922-1.00009-9
Keywords: light, plant defense, insect-borne disease, tripartite interaction, LEDs
Citation: Wang D, Dawadi B, Qu J and Ye J (2022) Light-Engineering Technology for Enhancing Plant Disease Resistance. Front. Plant Sci. 12:805614. doi: 10.3389/fpls.2021.805614
Received: 30 October 2021; Accepted: 31 December 2021;
Published: 17 February 2022.
Edited by:
Jigang Li, China Agricultural University, ChinaReviewed by:
Xian-Bing Wang, China Agricultural University, ChinaFang Lin, Lanzhou University, China
Copyright © 2022 Wang, Dawadi, Qu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Ye, jianye@im.ac.cn
 Duan Wang1,2
Duan Wang1,2 Jian Ye
Jian Ye