Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review
Abstract
:1. Introduction
1.1. Environmental Degradation and Pollution
1.2. Phytoremediation
1.3. Emerging Tools to Improve Plant Efficiency in Phytoremediation Programs
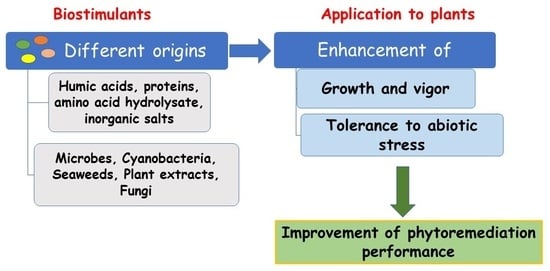
2. Plant Biostimulants
PBs in Helping Crops Cope with Toxic Compounds
3. PBs for Phytoremediation
3.1. PBs Derived from Humic Substances
3.2. Protein and Amino Acid Hydrolysate-Derived PBs
3.3. Inorganic Salt-Derived PBs
3.4. Microbial-Derived PBS
3.5. Seaweed-Derived PBS
3.6. Plant Extract-Derived PBs
3.7. Fungal PBS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasrollahi, Z.; Mohadeseh-Sadat, H.; Saeed, B.; Vahid, M.T. Environmental pollution, economic growth, population, industrialization, and technology in weak and strong sustainability: Using STIRPAT model. Environ. Dev. Sustain. 2020, 22, 1105–1122. [Google Scholar] [CrossRef]
- Destek, M.A.; Sarkodie, S.A. Investigation of environmental Kuznets curve for ecological footprint: The role of energy and financial development. Sci. Total Environ. 2019, 650, 2483–2489. [Google Scholar] [CrossRef]
- Esso, L.J.; Keho, Y. Energy consumption, economic growth and carbon emissions: Cointegration and causality evidence from selected African countries. Energy 2016, 114, 492–497. [Google Scholar] [CrossRef]
- Ozcan, B.; Tzeremes, P.G.; Tzeremes, N.G. Energy consumption, economic growth and environmental degradation in OECD countries. Econ. Model. 2020, 84, 203–213. [Google Scholar] [CrossRef]
- Rahman, M.M. Environmental degradation: The role of electricity consumption, economic growth and globalisation. J. Environ. Manag. 2020, 253, 109742. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Garg, N.; Paudel, R. Environmental degradation: Causes and consequences. Eur. Res. 2014, 81, 1491–1498. [Google Scholar]
- Ashraf, M.A.; Hanafiah, M.M. Sustaining life on earth system through clean air, pure water, and fertile soil. Environ. Sci. Pollut. Res. 2019, 26, 13679–13680. [Google Scholar] [CrossRef] [Green Version]
- Panfili, I.; Bartucca, M.L.; Ballerini, E.; Del Buono, D. Combination of aquatic species and safeners improves the remediation of copper polluted water. Sci. Total Environ. 2017, 601–602, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Shafiq, M.; Bareen, F.E. Fungal biostimulant-driven phytoextraction of heavy metals from tannery solid waste contaminated soils. Int. J. Phytoremediat. 2022, 24, 47–58. [Google Scholar] [CrossRef]
- Song, Z.; Shan, B.; Tang, W.; Zhang, C. Will heavy metals in the soils of newly submerged areas threaten the water quality of Danjiangkou Reservoir, China? Ecotoxicol. Environ. Saf. 2017, 144, 380–386. [Google Scholar] [CrossRef]
- Arunakumara, K.K.I.U.; Walpola, B.C.; Yoon, M.H. Current status of heavy metal contamination in Asia’s rice lands. Rev. Environ. Sci. Biotechnol. 2013, 12, 355–377. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Cui, L.; Wu, Y.; Fu, H.; Chen, J.; Chen, M. Atmospheric emissions of Cu and Zn from coal combustion in China: Spatio-temporal distribution, human health effects, and short-term prediction. Environ. Pollut. 2017, 229, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.U.R.; Ahmad, N.; Masood, K.R.; Peralta-Videa, J.R. Heavy metal toxicity in plants. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 71–98. [Google Scholar]
- Bartucca, M.L.; Celletti, S.; Mimmo, T.; Cesco, S.; Astolfi, S.; Del Buono, D. Terbuthylazineinterferes with ironnutrition in maize (Zeamays) plants. Acta Physiol. Plant. 2017, 39, 235. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Mimmo, T.; Cesco, S.; Panfili, I.; Del Buono, D. Effect of metribuzin on nitrogenmetabolism and iron acquisition in Zea mays. Chem. Ecol. 2019, 35, 720–731. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Astolfi, S.; Mimmo, T.; Bartucca, M.L.; Celletti, S.; Ciaffi, M.; Cesco, S. Effects of terbuthylazine on phytosiderophores release in iron deficient barley. Environ. Exp. Bot. 2015, 116, 32–38. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Del Buono, D. Effect of agrochemicals on biomass production and quality parameters of tobacco plants. J. Plant Nutr. 2020, 44, 1107–1119. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Di Michele, A.; Del Buono, D. Interference of three herbicides on iron acquisition in maize plants. Chemosphere 2018, 206, 424–431. [Google Scholar] [CrossRef]
- Del Buono, D.; Terzano, R.; Panfili, I.; Bartucca, M.L. Phytoremediation and detoxification of xenobiotics in plants: Herbicide-safeners as a tool to improve plant efficiency in the remediation of polluted environments. A mini-review. Int. J. Phytoremediat. 2020, 22, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.S.; Mahdi, A.J.; Bilal, M.; Sohail, H.M.; Ali, N.; Iqbal, H.M. Environmental impact and pollution-related challenges of renewable wind energy paradigm–a review. Sci. Total Environ. 2019, 683, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, R.; Singh, A.P.; Dhadse, K.; Garg, C. An evidence based integrated watershed modelling system to assess the impact of non-point source pollution in the riverine ecosystem. J. Clean. Prod. 2020, 246, 118963. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The treatment of duckweed with a plant biostimulant or a safener improves the plant capacity to clean water polluted by terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef]
- Lima, A.T.; Hofmann, A.; Reynolds, D.; Ptacek, C.J.; Van Cappellen, P.; Ottosen, L.M.; Pamukcu, S.; Alshawabekh, A.; O’Carroll, D.M.; Riis, C. Environmental electrokinetics for a sustainable subsurface. Chemosphere 2017, 181, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Phytoremediation potential of maize (Zea mays L.). A review. Afr. J. Agric. 2010, 6, 275–287. [Google Scholar]
- Dhanwal, P.; Kumar, A.; Dudeja, S.; Chhokar, V.; Beniwal, V. Recent advances in phytoremediation technology. Adv. Environ. Biotechnol. 2017, 227–241. [Google Scholar] [CrossRef]
- Gunarathne, V.; Mayakaduwa, S.; Ashiq, A.; Weerakoon, S.R.; Biswas, J.K.; Vithanage, M. Transgenic plants: Benefits, applications, and potential risks in phytoremediation. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Hyderabad: Hyderabad, India; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–102. [Google Scholar] [CrossRef]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis. Clean Soil Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- El Aafi, N.; Brhada, F.; Dary, M.; Maltouf, A.F.; Pajuelo, E. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant Rhizobacterium serratia sp. MSMC451. Int. J. Phytoremediat. 2012, 14, 261–274. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Hill, R.T.; Shukla, P. Bioremediation through microbes: Systems biology and metabolic engineering approach. Crit. Rev. Biotechnol. 2019, 39, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bharadvaja, N. Enzymatic bioremediation: A smart tool to fight environmental pollutants. In Smart Bioremediation Technologies; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 99–118. [Google Scholar]
- Pannacci, E.; Del Buono, D.; Bartucca, M.L.; Nasini, L.; Proietti, P.; Tei, F. Herbicide uptake and regrowth ability of tall fescue and orchardgrass in s-metolachlor-contaminated leachates from sand pot experiment. Agriculture 2020, 10, 487. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Mimmo, T.; Cesco, S.; Del Buono, D. Nitrate removal from polluted water by using a vegetated floating system. Sci. Total Environ. 2016, 542, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Pannacci, E.; Bartucca, M.L.; Nasini, L.; Proietti, P.; Tei, F. Use of two grasses for the phytoremediation of aqueous solutions polluted with terbuthylazine. Int. J. Phytoremediat. 2016, 18, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Bartucca, M.L.; Del Buono, D.; Cesco, S. Italian ryegrass for the phytoremediation of solutions polluted with terbuthylazine. Chemosphere 2015, 119, 31–36. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Ann. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Farraji, H.; Zaman, N.Q.; Tajuddin, R.; Faraji, H. Advantages and disadvantages of phytoremediation: A concise review. Int. J. Environ. Technol. Sci. 2016, 2, 69–75. [Google Scholar]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal—contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Farraji, H.; Robinson, B.; Mohajeri, P.; Abedi, T. Phytoremediation: Green technology for improving aquatic and terrestrial environments. Nippon. J. Environ. Sci. 2020, 1, 1–30. [Google Scholar] [CrossRef]
- Baştabak, B.; Gödekmerdan, E.; Koçar, G. A holistic approach to soil contamination and sustainable phytoremediation with energy crops in the Aegean Region of Turkey. Chemosphere 2021, 276, 130192. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Nandita, S.; Tuli, R.; Gupta, D.K.; Maathuis, F.J.M. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Pilbeam, D.; Inal, A. Effect of arsenic-phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil. 2009, 314, 211–220. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T.; Okumura, C.; Rahman, M.M. Arsenic accumulation in duckweed (Spirodela polyrhiza L.): A good option for phytoremediation. Chemosphere 2007, 69, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.I.; Zimmerman, A.R.; Kim, J.E. Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants. Sci. Total Environ. 2018, 627, 514–522. [Google Scholar] [CrossRef]
- Daud, M.K.; Ali, S.; Abbas, Z.; Zaheer, I.E.; Riaz, M.A.; Malik, A.; Hussain, A.; Rizwan, M.; Zia-ur-Rehman, M.; Zhu, S.J. Potential of duckweed (Lemna minor) for the phytoremediation of landfill leachate. J. Chem. 2018, 2018, 3951540. [Google Scholar] [CrossRef] [Green Version]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef]
- Ekta, P.; Modi, N.R. A review of phytoremediation. J. Pharm. Phytochem. 2018, 7, 1485–1489. [Google Scholar]
- El-Aassar, M.R.; Fakhry, H.; Elzain, A.A.; Farouk, H.; Hafez, E.E. Rhizofiltration system consists of chitosan and natural Arundo donax L. for removal of basic red dye. Int. J. Biol. Macromol. 2018, 120, 1508–1514. [Google Scholar] [CrossRef]
- Zahoor, M.; Irshad, M.; Rahman, H.; Qasim, M.; Afridi, S.G.; Qadir, M.; Hussain, A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017, 142, 139–149. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation potential of fast-growing energy plants: Challenges and perspectives—A review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, V.L.; Cummins, I.; Brazier-Hicks, M.; Edwards, R. Protective responses induced by herbicide safeners in wheat. Env. Exp. Bot. 2013, 88, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Bartucca, M.L.; Celletti, S.; Astolfi, S.; Mimmo, T.; Cesco, S.; Panfili, I.; Del Buono, D. Effect of three safeners on sulfur assimilation and iron deficiency response in barley (Hordeum vulgare) plants. Pest Manag. Sci. 2017, 73, 240–245. [Google Scholar] [CrossRef]
- Sivey, J.D.; Lehmler, H.-J.; Salice, C.J.; Ricko, A.N.; Cwiertny, D.M. Environmental fate and effects of dichloroacetamide herbicide safeners: ‘inert’ yet biologically active agrochemical ingredients. Environ. Sci. Technol. Lett. 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, N. Transgenic plants for phytoremediation. Int. J. Phytoremediat. 2011, 13 (Suppl. 1), 264–279. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Pilon-Smits, E.A.; Meagher, R.B.; Doty, S. Biotechnological approaches for phytoremediation. In Plant Biotechnology and Agriculture; Oxford Academic Press: New York, NY, USA, 2011; pp. 309–328. [Google Scholar]
- Rai, P.K.; Kim, K.H.; Lee, S.S.; Lee, J.H. Molecular mechanisms in phytoremediation of environmental contaminants and prospects of engineered transgenic plants/microbes. Sci. Total Environ. 2020, 705, 135858. [Google Scholar] [CrossRef]
- Bell, T.H.; Joly, S.; Pitre, F.E.; Yergeau, E. Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol. 2014, 32, 271–280. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Ma, D.; Ma, F.; Zhilong, B. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 109355. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- La Torre, A.; Battaglia, V.; Caradonia, F. An overview of the current plant biostimulant legislations in different European Member States. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Buono, D.; Regni, L.; Del Pino, A.M.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of megafol on the olive cultivar ‘Arbequina’ grown under severe saline stress in terms of physiological traits, oxidative stress, antioxidant defenses, and cytosolic Ca2+. Front. Plant Sci. 2020, 11, 603576. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.; Irineu, L.E.S.D.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; Desoky, E.S.M.; Rady, M.M. Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a plant biostimulant to improve maize (Zea mays) tolerance to metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Constantin, J.; de Oliveira, R.S.E., Jr.; Gheno, E.A.; Biffe, D.F.; Braz, G.B.P.; Weber, F.; Takano, H.K. Prevention of yield losses caused by glyphosate in soybeans with biostimulant. Afr. J. Agric. Res. 2016, 11, 1601–1607. [Google Scholar]
- Santos, A.; Freitas, F.C.L.; Santos, I.T.; Silva, D.C.; Alcantara-De la Cruz, R.; Ferreira, L.R. Use of Fertiactyl Pós® for protection of Eucalyptus plants subjected to herbicide drift. Planta Daninha 2020, 38, e020176980. [Google Scholar] [CrossRef]
- Balabanova, D.A.; Paunov, M.; Goltsev, V.; Cuypers, A.; Vangronsveld, J.; Vassilev, A. Photosynthetic performance of the imidazolinone resistant sunflower exposed to single and combined treatment by the herbicide imazamox and an amino acid extract. Front. Plant Sci. 2016, 7, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada, M.; García-Martínez, A.M.; Gómez, I.; Parrado, J. Application of MCPA herbicide on soils amended with biostimulants: Short-time effects on soil biological properties. Chemosphere 2010, 80, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morgado, B.; Gómez, I.; Parrado, J.; Tejada, M. Behaviour of oxyfluorfen in soils amended with edaphic biostimulants/biofertilizers obtained from sewage sludge and chicken feathers. Effects on soil biological properties. Environ. Sci. Pollut. Res. 2014, 21, 11027–11035. [Google Scholar] [CrossRef]
- Kanissery, R.G.; Sims, G.K. Biostimulation for the enhanced degradation of herbicides in soil. Appl. Environ. Soil Sci. 2011, 2011, 843450. [Google Scholar] [CrossRef] [Green Version]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Berbara, R.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Mosa, A.A.; Taha, A.; Elsaeid, M. Agro-environmental applications of humic substances: A critical review. Egypt. J. Soil Sci. 2020, 60, 207–220. [Google Scholar] [CrossRef]
- Pittarello, M.; Busato, J.G.; Carletti, P.; Sodré, F.F.; Dobbss, L.B. Dissolved humic substances supplied as potential enhancers of Cu, Cd, and Pb adsorption by two different mangrove sediments. J. Soils Sediment 2019, 19, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Pittarello, M.; Busato, J.G.; Carletti, P.; Zanetti, L.V.; da Silva, J.; Dobbss, L.B. Effects of different humic substances concentrations on root anatomy and Cd accumulation in seedlings of Avicennia germinans (black mangrove). Mar. Pollut. Bull. 2018, 130, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dobbss, L.B.; Dos Santos, T.C.; Pittarello, M.; de Souza, S.B.; Ramos, A.C.; Busato, J.G. Alleviation of iron toxicity in Schinus terebinthifolius Raddi (Anacardiaceae) by humic substances. Environ. Sci. Pollut. Res. 2018, 25, 9416–9425. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Rocco, C.; Cenvinzo, V.; Agrelli, D.; Gioia, L.; Di Mola, I.; Adamo, P.; Pepe, O.; Fagnano, M. Giant reed growth and effects on soil biological fertility in assisted phytoremediation of an industrial polluted soil. Sci. Total Environ. 2017, 575, 1375–1383. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Kim, K.S.; Park, S. Enhancing degradation of total petroleum hydrocarbons and uptake of heavy metals in a wetland microcosm planted with Phragmites communis by humic acids addition. Int. J. Phytoremediat. 2013, 15, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, M.; Mosca, G.; Vamerali, T. Humic acids affect root characteristics of fodder radish (Raphanus sativus L. var. oleiformis Pers.) in metal-polluted wastes. Desalination 2009, 246, 78–91. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.; Stewart, R.B.; Robinson, B.H.; Ghomshei, M.; Meech, J.A. Induced plant uptake and transport of mercury in the presence of sulphur-containing ligands and humic acid. New Phytol. 2005, 166, 445–454. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Ugolini, L.; Cinti, S.; Righetti, L.; Stefan, A.; Matteo, R.; D’Avino, L.; Lazzeri, L. Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind. Crops Prod. 2015, 75, 15–23. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Olaifa, F.E.; Omekam, A.J. Studies on phytoremediation of copper using Pteridium aquilinum (bracken fern) in the presence of biostimulants and bioassay using Clarias gariepinus juveniles. Int. J. Phytoremediat. 2014, 16, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Ndimele, P.E.; Jenyo-Oni, A.; Chukwuka, K.S.; Ndimele, C.C.; Ayodele, I.A. Does fertilizer (N15P15K15) amendment enhance phytoremediation of petroleum-polluted aquatic ecosystem in the presence of water Hyacinth (Eichhornia crassipes [Mart.] Solms)? Environ. Technol. 2015, 36, 2502–2514. [Google Scholar] [CrossRef]
- Guo, J.M.; Lei, M.; Yang, J.X.; Yang, J.; Wan, X.M.; Chen, T.-B.; Zhou, X.-Y.; Gu, S.-P.; Guo, G.H. Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol. Eng. 2017, 106, 409–414. [Google Scholar] [CrossRef]
- Zhou, G.; Guo, J.; Yang, J. Effect of fertilizers on Cd accumulation and subcellular distribution of two cosmos species (Cosmos sulphureus and Cosmos bipinnata). Int. J. Phytoremediat. 2018, 20, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Chi, Y.; Chu, S.; Hayat, K.; Zhi, Y.; Hayat, S.; Terziev, D.; Zhang, D.; Zhou, P. Optimization of NPK fertilization combined with phytoremediation of cadmium contaminated soil by orthogonal experiment. Ecotoxicol. Environ. Saf. 2020, 189, 109997. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Rusinowski, S.; Sitko, K.; Krzyzak, J.; Skalska, A.; Malkowski, E.; Ciszek, D.; Werle, S.; McCalmont, J.P.; Mos, M.; et al. Relationships between soil parameters and physiological status of Miscanthus × giganteus cultivated on soil contaminated with trace elements under NPK fertilisation vs. microbial inoculation. Environ. Pollut. 2017, 225, 163–174. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; Rios, J.J.; Blasco, B.; Ruiz, J.M. Assaying the use of sodium thiosulphate as a biostimulant and its effect on cadmium accumulation and tolerance in Brassica oleracea plants. Ecotoxicol. Environ. Saf. 2020, 200, 110760. [Google Scholar] [CrossRef]
- Grifoni, M.; Schiavon, M.; Pezzarossa, B.; Petruzzelli, G.; Malagoli, M. Effects of phosphate and thiosulphate on arsenic accumulation in the species Brassica juncea. Environ. Sci. Pollut. Res. 2015, 22, 2423–2433. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Ding, S.; Xiao, H. Enhancer assisted-phytoremediation of mercury contaminated soils by Oxalis corniculata L., and rhizosphere microorganism distribution of Oxalis corniculata L. Ecotoxi. Environ. Saf. 2018, 160, 171–177. [Google Scholar] [CrossRef]
- Ondrasek, G.; Romic, D.; Rengel, Z. Interactions of humates and chlorides with cadmium drive soil cadmium chemistry and uptake by radish cultivars. Sci. Total Environ. 2020, 702, 134887. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Abdal, N.; Abbas, G.; Asad, S.A.; Ghfar, A.A.; Shah, G.M.; Rizwan, M.; Ali, S.; Shahbaz, M. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: Implications for phytoremediation. Environ. Geochem. Health, 2021; Epub ahead of print. [Google Scholar] [CrossRef]
- Radziemska, M.; Bęś, A.; Gusiatin, Z.M.; Sikorski, Ł.; Brtnicky, M.; Majewski, G.; Liniauskienė, E.; Pecina, V.; Datta, R.; Bilgin, A.; et al. Successful outcome of phytostabilization in Cr(VI) contaminated soils amended with alkalizing additives. Int. J. Environ. Res. Public Health 2020, 17, 6073. [Google Scholar] [CrossRef]
- Ahmad, I.; Pichtel, J.; Hayat, S. Plant-Bacteria Interactions. Strategies and Techniques to Promote Plant Growth; Wiley-VCH Verlag GmbH & Co. Germany: Weinheim, Germany, 2008. [Google Scholar]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J. Appl. Microbiol. 2009, 108, 236–245. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Rossi, M.; D’Egidio, S.; Del Gallo, M.; Forni, C. Daucus carota L. seed inoculation with a consortium of bacteria improves plant growth and soil fertility status and microbial community. Appl. Sci. 2021, 11, 3274. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Paço, A.; da-Silva, J.R.; Pereira Torres, D.; Glick, B.R.; Brígido, C. Exogenous ACC deaminase is key to improving the performance of pasture legume-rhizobial symbioses in the presence of a high manganese concentration. Plants 2020, 9, 1630. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Li, X.; Li, T.; He, X.; Tao, Y. Effects of Pseudomonas chenduensis and biochar on cadmium availability and microbial community in the paddy soil. Sci. Total Environ. 2018, 640, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mou, R.; Cao, Z.; Xu, P.; Wu, X.; Zhu, Z.; Chen, M. Characterization of cadmium-resistant bacteria and their potential for reducing accumulation of cadmium in rice grains. Sci. Total Environ. 2016, 569–570, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Kumar Ghosh, P.; Soren, T.; Kanti Maiti, T. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Krishnamurthi, K.; Patil, M.; Chakrabarti, T. Heavy metal resistance in Arthrobacter ramosus strain G2 isolated from mercuric salt-contaminated soil. J. Hazard. Mater. 2010, 177, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Zhao, B.; Xin, X.; Zhang, C.; Zhang, H. The influence of long-term fertilization on cadmium (Cd) accumulation in soil and its uptake by crops. Environ. Sci. Pollut. Res. 2014, 21, 10377–10385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Yu, Z.; Wang, C.H. Bioaccumulation of cadmium in the ascidian Styela clava (Herdman 1881). Afr. J. Mar. Sci. 2009, 31, 289–295. [Google Scholar] [CrossRef]
- Reddy, K.R.; Chinthamreddy, S.; Saichek, R.E. Nutrient amendment for the bioremediation of a Chromium-contaminated soil by electrokinetics. Energy Sources 2003, 25, 931–943. [Google Scholar] [CrossRef]
- Backer, R.; Rockem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. PGPR: Biostimulants for sustainable agriculture and promoting early colonization of the rhizosphere with beneficial microorganisms. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Haegi, A.; Del Gallo, M.; Grilli Caiola, M. Production of polysaccharides by Arthrobacter globiformis associated with Anabaena azollae in Azolla leaf cavity. FEMS Microbiol. Lett. 1992, 93, 269–274. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Diwan, P. Bacterial Exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J. Cyanobacteria in plant health: Biological strategy against abiotic and biotic stresses. Crop Prot. 2021, 14, 105450. [Google Scholar] [CrossRef]
- Garlapati, D.; Chandrasekaran, M.; Devanesan, A.; Mathimani, T.; Pugazhendhi, A. Role of cyanobacteria in agricultural and industrial sectors: An outlook on economically important byproducts. Appl. Microbiol. Biotechnol. 2019, 103, 4709–4721. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Goyal, D. Heavy metal accumulation from coal fly ash by cyanobacterial biofertilizers. Part. Sci. Technol. 2018, 36, 513–516. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [Green Version]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011, 92, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2009, 17, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Hassani, S.B.; Aliniaeifard, S. Seed priming by Cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against Cadmium toxicity. J. Plant Growth Regul. 2019, 39, 1009–1021. [Google Scholar] [CrossRef]
- Faisal, M.; Hameed, A.; Hasnain, S. Chromium-resistant bacteria and cyanobacteria: Impact on Cr (VI) reduction potential and plant growth. J. Ind. Microbiol. Biotechnol. 2005, 32, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Aminfarzaneh, H.; Duygu, E. The effect of salicylic acid and triacontanol on biomass production andimidaclopirid removal capacity by cyanobacteria. Commun. Fac. Sci. Univ. Ank. Ser. 2010, 22, 15–31. [Google Scholar]
- Tiwari, B.; Singh, S.; Chakraborty, S.; Verma, E.; Mishra, A.K. Sequential role of biosorption and biodegradation in rapid removal degradation and utilization of methyl parathion as a phosphate source by a new cyanobacterial isolate Scytonema sp. BHUS-5. Int. J. Phytoremediat. 2017, 19, 884–893. [Google Scholar] [CrossRef]
- Tiwari, B.; Chakraborty, S.; Srivastava, A.K.; Mishra, A.K. Biodegradation and rapid removal of methyl parathion by the paddy field cyanobacterium Fischerella sp. Algal Res. 2017, 25, 285–296. [Google Scholar] [CrossRef]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Mourad, B. The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Mustapha, M.U.; Halimoon, N. Microorganisms and biosorption of heavy metals in the environment: A review paper. J. Microb. Biochem. Technol. 2015, 7, 253–256. [Google Scholar] [CrossRef]
- Shekhar Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Trejo Valencia, R.; Sánchez Acosta, L.; Fortis Hernández, M.; Preciado Rangel, P.; Gallegos Robles, Á.M.; Antonio Cruz, D.R.; Vázquez Vázquez, C. Effect of seaweed aqueous extracts and compost on vegetative growth, yield, and nutraceutical quality of cucumber (Cucumis sativus L.) fruit. Agronomy 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Stirk, W.A.; Novak, O.; Hradecka, V.; Pencik, A.; Rolcik, J.; Strnad, M.; Van Staden, J. Endogenous cytokinins, auxins and abscisic acid in Ulva fasciata (Chlorophyta) and Dictyotahumifusa (Phaeophyta): Towards understanding their biosynthesis and homoeostasis. Eur. J. Phycol. 2009, 44, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Zerrifi, S.E.A.; El Khalloufi, F.; Oudra, B.; Vasconcelos, V. Seaweed bioactive compounds against pathogens and microalgae: Potential uses on pharmacology and harmful algae bloom control. Mar. Drugs 2018, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Wang, H.; Liu, Q.; Lin, L.; Liao, M.A.; Deng, H.; Wang, Z.; Liang, D.; Wang, X.; Xia, H.; et al. An algal biostimulant promotes growth and decreases cadmium uptake in accumulator plant Nasturtium officinale. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Saa, S.; Rio, O.D.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] DA Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Khan, M.A.; Luo, W.; Xiang, Z.; Xu, W.; Zhong, B.; Ma, J.; Ye, Z.; Zhu, Y.; et al. Effect of plant extracts and citric acid on phytoremediation of metal-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 211, 111902. [Google Scholar] [CrossRef] [PubMed]
- Heaton, E.A.; Dohleman, F.G.; Miguez, A.F.; Juvik, J.A.; Lozovaya, V.; Widholm, J.; Zabotina, O.A.; Mcisaac, G.F.; David, M.B.; Voigt, T.B.; et al. Miscanthus: A promising biomass crop. In Advances in Botanical Research; Academic Press: New York, NY, USA, 2010; Volume 56, pp. 75–137. [Google Scholar]
- Técher, D.; Laval-Gilly, P.; Henry, S.; Bennasroune, A.; Formanek, P.; Martinez-Choice, C.; D’Innocenzo, M.; Muanda, F.; Dicko, A.; Rejšek, K.; et al. Contribution of Miscanthus × giganteus root exudates to the biostimulation of PAH degradation: An in vitro study. Sci. Total Environ. 2011, 409, 4489–4495. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Baldassarre Švecová, E.; Ruzzi, M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Arbuscular mycorrhizal networks: Process and function. A review. Agron. Sustain. Dev. 2010, 30, 581–599. [Google Scholar] [CrossRef] [Green Version]
- Asemoloye, M.D.; Jonathan, S.G.; Jayeola, A.A.; Ahmad, R. Mediational influence of spent mushroom compost on phytoremediation of black-oil hydrocarbon polluted soil and response of Megathyrsus maximus. J. Environ. Manag. 2017, 200, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Asemoloye, M.D.; Chukwuka, K.S. Spent mushroom compost enhances plant response and phytoremediation of heavy metal polluted soil. J. Plant Nutr. Soil Sci. 2020, 183, 492–499. [Google Scholar] [CrossRef]

| Advantages | Disadvantages |
|---|---|
| Suitable for various types of contaminants (organic substances, metals, metalloids, dyes, hydrocarbons, radioactive substances) | Not applicable in some circumstances (for example, when contaminants are found in deep soil layers, not accessible to the roots) |
| Efficient | Contaminants cannot be completely removed |
| Relatively cheap | Slower than conventional methods |
| Environmentally friendly | Not convenient for heavily polluted sites (due to the limited tolerance of a plant to pollutants) |
| Non-destructive | Difficult to apply when the pollutant is not completely bioavailable |
| Non-invasive | Strictly dependent on the environmental conditions |
| Aesthetically pleasing | The handling and disposal of harvested plant tissues could be problematic |
| Directly applicable in situ | Still under development (its potential has not been fully exploited) |
| Does not require energy | Commercial-scale applications of this technology are few and still inadequate |
| Can be used to remove more than one pollutant at the same time | |
| Has minimal equipment requirements | |
| Can be combined with other methods, such as conventional technologies | |
| Contaminants can be recovered from the plant tissues and marketed | |
| Provides habitats for animals | |
| Stimulates beneficial microbes | |
| Reduces soil erosion, simultaneously improving its structure and fertility | |
| Contributes to carbon sequestration |
| Plant Species | PB | Pollutant | PB Recommended Dose | Results | Ref. |
|---|---|---|---|---|---|
| Maize | Humic substances | Cr | 4 mM C HA L−1 |
| [79] |
| Maize | Silymarin-based biostimulant | Cd | 0.24 g L−1 |
| [82] |
| Maize | Megafol | Metolachlor | 2.5 L ha−1 |
| [83] |
| Soybean | Fertiacyl Pòs | Glyphosate | 0.4 L ha−1 |
| [84] |
| Sunflower | Protein hydrolysates | Imazamox | 3 L ha−1 |
| [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review. Plants 2022, 11, 1946. https://doi.org/10.3390/plants11151946
Bartucca ML, Cerri M, Del Buono D, Forni C. Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review. Plants. 2022; 11(15):1946. https://doi.org/10.3390/plants11151946
Chicago/Turabian StyleBartucca, Maria Luce, Martina Cerri, Daniele Del Buono, and Cinzia Forni. 2022. "Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review" Plants 11, no. 15: 1946. https://doi.org/10.3390/plants11151946